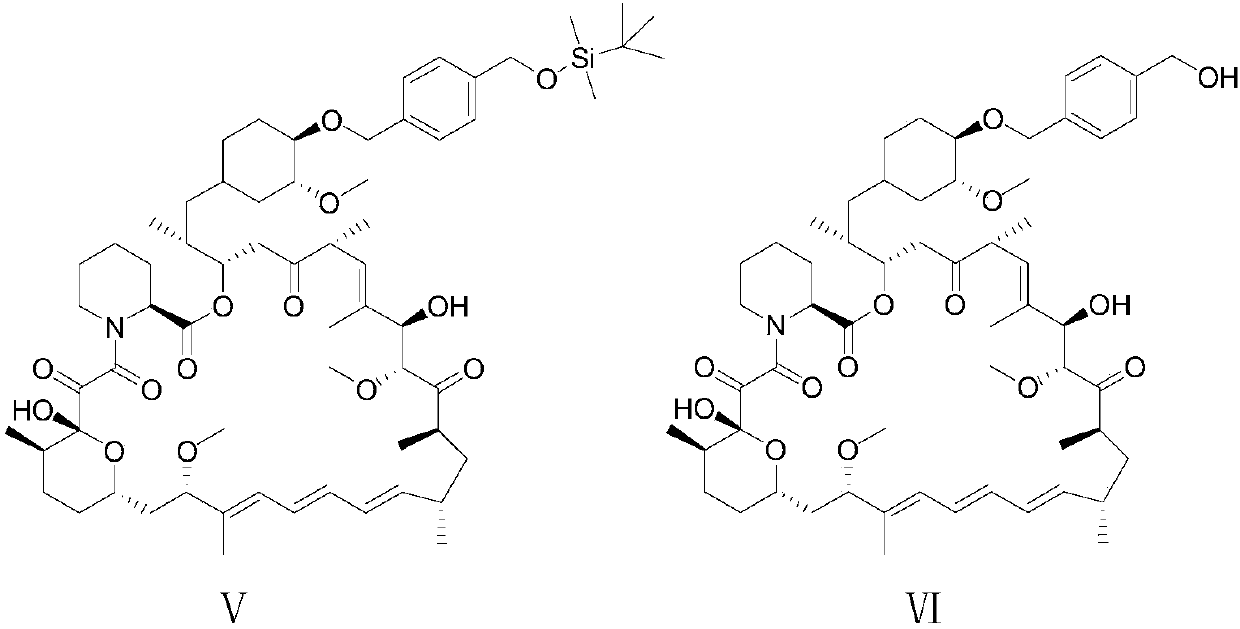
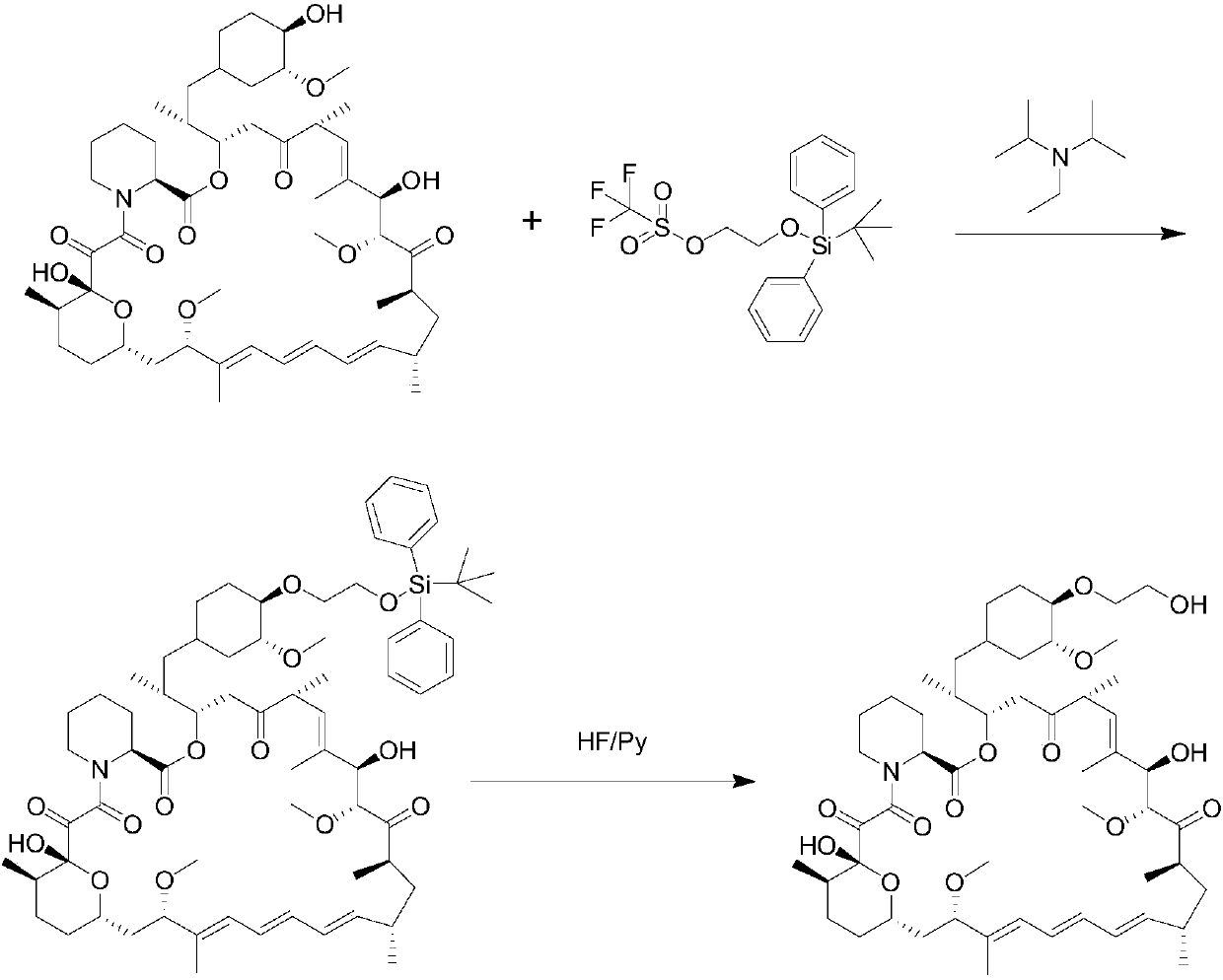
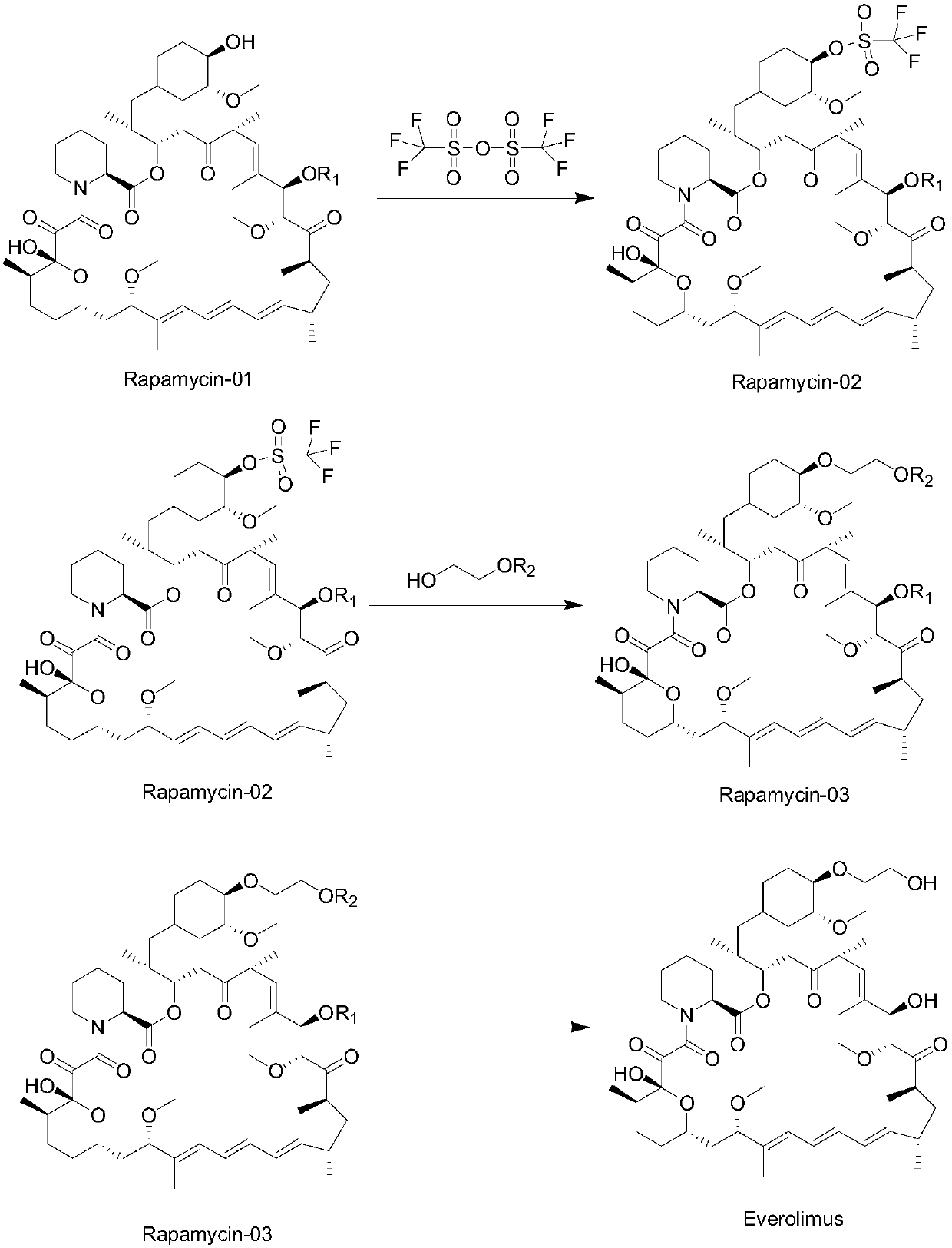
A kind of synthetic method of sirolimus 40-ether derivative
A technique for the synthesis of sirolimus and a synthetic method, which is applied in the field of synthesis of sirolimus 40-ether derivatives, can solve problems such as inability to preserve, low reaction yield, and instability, and achieve simplified production processes and reaction steps , to avoid troublesome effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Synthesis of 40-O-(2-methoxyethyl)-sirolimus
[0037] In a 100ml three-necked flask, add sirolimus 2.0g (2.2mmol), 4-dimethylaminopyridine 2.8g, silver trifluoromethanesulfonate 5.9g (23.0mmol MW: 256.9), 1-iodo-2- 4.3g of methoxyethane (23.1mmol MW: 186.0) and 20ml of isopropyl acetate were stirred at 0-10°C for 24 hours to complete the reaction. The reaction solution was poured into an equal volume of saturated sodium chloride solution for extraction, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a brown liquid. Separation by silica gel column chromatography, using ethyl acetate as a developing solvent, and rotary evaporation to obtain 1.5 g of 40-O-(2-methoxyethyl)-sirolimus (yield: 70.2%). HPLC detection showed that the purity was 86.8%.
Embodiment 2
[0038] Example 2. Synthesis of 40-O-(2-tert-butyldiphenylsilaneethyl)-sirolimus
[0039] Synthesis of halogen compounds: In a 100ml three-necked flask, add 5ml of 2-iodoethanol, 40ml of ethyl acetate, add 5.5g of imidazole at room temperature, dropwise add 22.0g of tert-butyldiphenylchlorosilane, and react until 2-iodo After the ethanol reaction is complete, wash twice with saturated sodium chloride solution, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain a tert-butyl-(2-iodoethoxy)diphenylsilane intermediate.
[0040] Synthesis of 40-O-(2-tert-butyldiphenylsilaneethyl)-sirolimus: In a 100ml three-necked flask, add 2.0g (2.2mmol) sirolimus, 40ml dichloromethane, 2 , 3ml of 6-lutidine, 2.8g (11.0mmol) of silver trifluoromethanesulfonate, 4.6g of tert-butyl (2-iodoethoxy) diphenylsilane, and the reaction was completed with stirring at 20-30°C for 48 hours. The reaction solution was poured into an equal volume of saturated sodium chloride solution and wash...
Embodiment 3
[0042] Example 3. Synthesis of 40-O-benzyl-sirolimus
[0043] Put 2g (2.2mmol) of sirolimus (2.2mmol), 50ml of toluene, 11.3g (44mmol) of silver trifluoromethanesulfonate, 7.5g of bromotoluene, and 1.5g of imidazole into a 100ml three-necked flask, and react at about 35-40°C for 48 hours. Finish. Filter first, filter out solid substances such as silver trifluoromethanesulfonate, wash once with 50ml of saturated sodium chloride, dry over anhydrous magnesium sulfate, filter, spin dry and pass through the column to obtain 40-O-benzyl-Ciro Moss 2g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com