Fusion protein of anthrax toxin receptor cmg2 and human serum albumin
An anthrax toxin receptor, human serum albumin technology, applied in the field of fusion proteins, can solve problems such as uncontrollable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of fusion protein (CMG2-HSA)
[0030] 1. Expression vector construction
[0031] The fusion protein (CMG2-HSA) expression sequence of CMG2 and albumin was constructed in three steps:
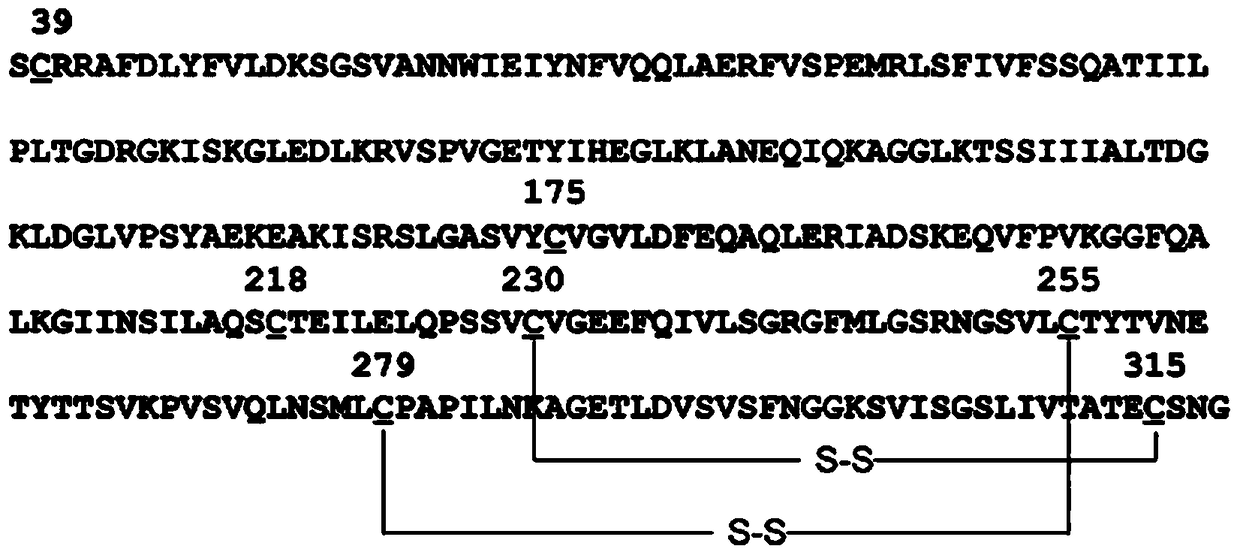
[0032] Step 1: Use primers H-1F and H-1R to amplify the HSA fragment from the plasmid containing the HSA coding sequence cDNA (for example, the T-HSA plasmid kept in our laboratory) by PCR method, and at the same time at its 5' end and An XhoI restriction site and a part of the linker sequence (linker) were added to the 3' end: GGTGGAGGCGGTTCAGGCGG. Use primers CMG2-F and CMG2-R to amplify the sCMG2 fragment from a plasmid containing the sCMG2 coding sequence (for example, PQE30-sCMG2 preserved in our laboratory) by PCR method, and add another part at its 5' end and 3' end, respectively Linker sequence (GTGGCTCTGGCGGTGGCGGATCG) and NotI restriction site. The above steps can also be accomplished by artificially synthesizing nucleotide sequences. Compared w...
Embodiment 2
[0042] Embodiment 2.SPR method detects the affinity of class receptor and PA
[0043] BiacoreT200 (GE) was used and completed at 25°C. Reagents used for coupling, conjugation, and regeneration were all from BIAcore. The reaction buffer is HBS-P+5mM MgCl 2 (10 mM Hepes, 0.15M NaCl, 0.005% P20, pH=7.4). At pH=4.0, 30 μg of PA was immobilized on the CM5 chip by amino coupling. 1 channel as reference channel, only use HBS-P+5mM MgCl 2 as the mobile phase. The regeneration buffer was 10 mM sodium tetraborate, 1 mol / L NaCl, pH=8.5, and all data analyzes were performed using BiacoreT200 evaluation software.
[0044] As shown in Figure 5, CMG2-HSA ( Figure 5A ), sCMG2 ( Figure 5B ) and PA were 3.9nmol / L and 1.915nmol / L respectively. Although CMG2-HSA was slightly lower than sCMG2 in terms of absolute value, it was still at a relatively high affinity level and could reach the nmol / L level.
Embodiment 3
[0045] Example 3. The metabolism of CMG2-HSA in rats
[0046] Two male SD rats (190-210 g, SCXK (Beijing) 2012-0001) were used to inject 100 μg of CMG2-HSA diluted with PBS through the tail vein. Blood was collected at different time points before and after injection until 6 days after injection, and blood was collected 8 times in total. Samples were collected in EP tubes treated with heparin, placed on ice, centrifuged at 4000×g for 15 minutes at 4°C, separated from plasma, and frozen at -20°C until use. The content of CMG2-HSA in the sample was measured by double-antibody sandwich ELISA, and the elimination half-life of CMG2-HSA in vivo was calculated. PBS was coated with 4 μg / mL anti-HSA monoclonal antibody (lifespan biosciences, LS-C51816) on overnight at ℃, wash 4 times with PBST (containing 0.2% Tween-20) the next day, 5 minutes each time; then block with 2% BSA at 37 ℃ for 1 h, repeat the washing step; Add the diluted sample to be tested, incubate at 37°C for 1 h, rep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com