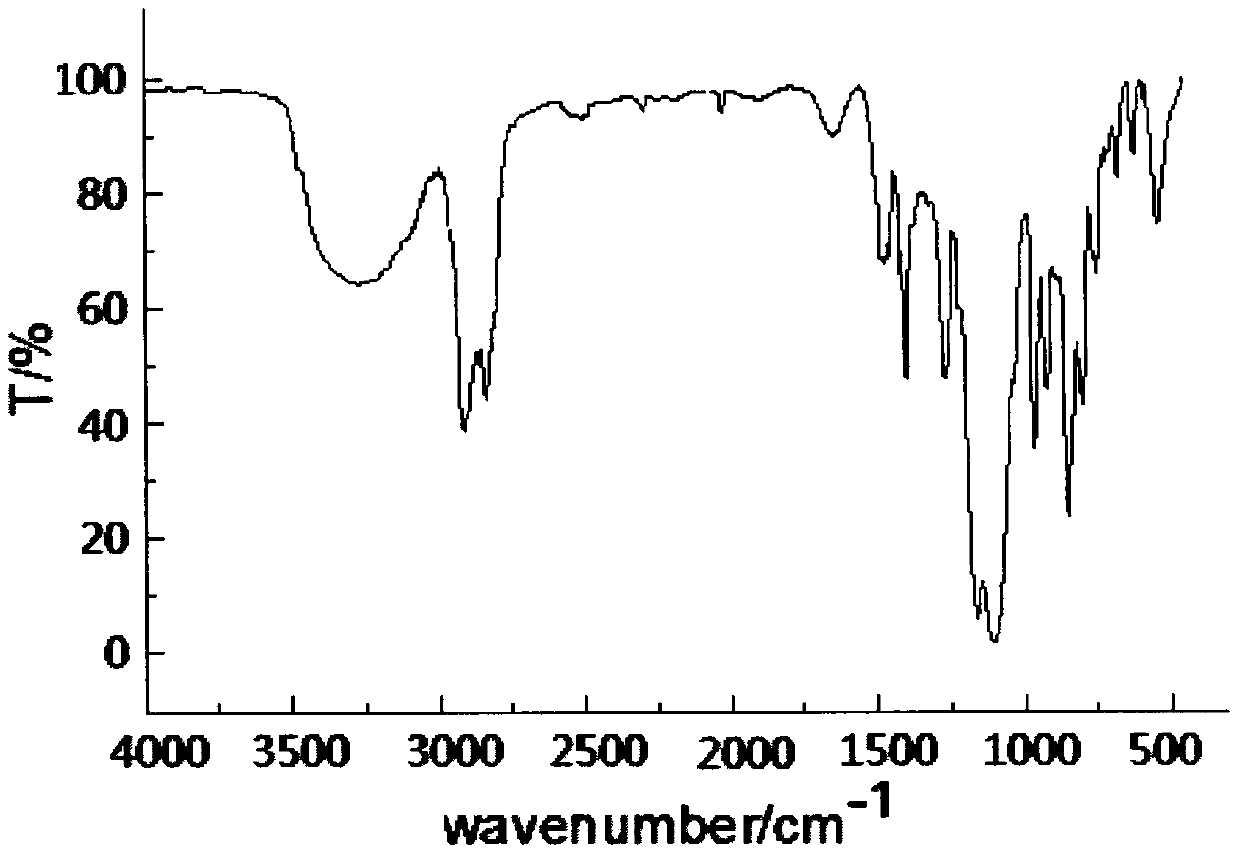
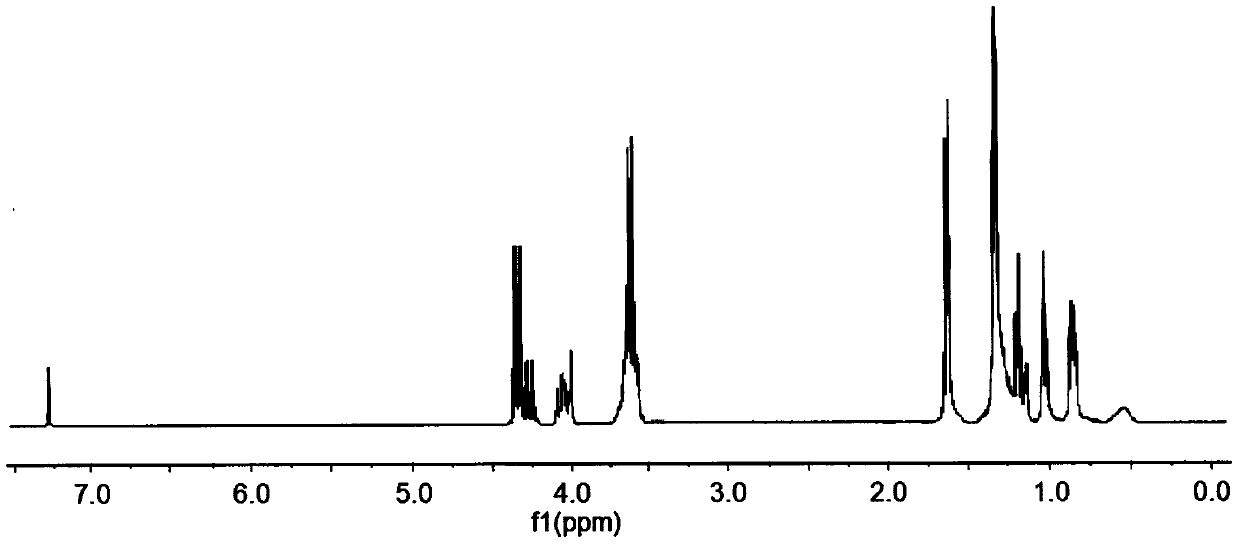
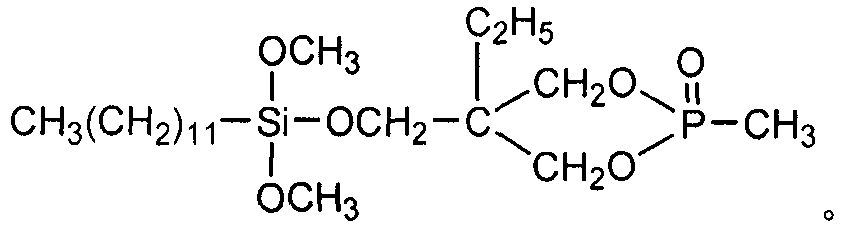
The preparation method of dodecyl dimethoxy (phosphacyclomethoxy) silane compound
A technology of dodecyl dimethoxy and silane compounds is applied in the field of preparation of flame retardant dodecyl dimethoxy silane compounds, which can solve the problem of slow development, poor electrical performance, and ease of use of silicone flame retardants. Migration and other problems, to achieve the effect of small mechanical properties, no pollution of three wastes, and stable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, the air in the flask was replaced with nitrogen, and 19.40g (0.10mol) 4-hydroxymethyl-4-ethyl-cyclomethan was added. Base phosphonate, 29.05g (0.10mol) dodecyltrimethoxysilane, 80ml diethylene glycol dimethyl ether, heat up to 140℃, control the top temperature of the fractionation column to be not higher than 65℃, and fractionate the methanol produced After reacting for 6h, when no methanol is produced, change to a vacuum distillation device, distill off diethylene glycol dimethyl ether (recycled) under reduced pressure, then add 50ml of toluene, stir for 20min, and transfer to a separatory funnel to stand still Separate the lower layer of the liquid, and distill under reduced pressure to remove a small amount of toluene and low boiling point substances to obtain a light yellow liquid dodecyl dimethoxy (phosphorheterocyclic methoxy) silane. The produc...
Embodiment 2
[0022] Example 2 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, the air in the flask was replaced with nitrogen, and 19.40g (0.10mol) 4-hydroxymethyl-4-ethyl-cyclomethan was added. Base phosphonate, 37.77g (0.13mol) dodecyltrimethoxysilane, 100ml ethylene glycol diethyl ether, heat up to 120℃, control the top temperature of the fractionation column to be not higher than 65℃, fractionate the methanol produced, and react After 9h, when no methanol is produced, change to a vacuum distillation device, distill out ethylene glycol diethyl ether under reduced pressure (recovered and used), then add 80ml of toluene, stir for 20min, transfer to a separatory funnel and let stand for layering. Take out the lower layer of liquid, and distill under reduced pressure to remove a small amount of toluene and low boiling point substances to obtain a light yellow liquid dodecyl dimethoxy (phosphorheterocyclic methoxy) silane. The pr...
Embodiment 3
[0023] Example 3 In a 200ml four-necked flask equipped with a stirrer, a thermometer and a high-efficiency fractionation device, the air in the flask was replaced with nitrogen, and 19.40g (0.10mol) 4-hydroxymethyl-4-ethyl-cyclomethan was added. Phosphonate, 40.67g (0.14mol) dodecyltrimethoxysilane, 60ml DMF, heat up to 130℃, control the top temperature of the fractionation column to be not higher than 65℃, fractionate the methanol produced, and react for 8h. After no methanol is produced, change to a vacuum distillation device, distill out DMF under reduced pressure (recovered and used), then add 70ml of toluene, stir for 20min, transfer to a separatory funnel, stand still for stratification, separate the lower layer of liquid, reduce pressure A small amount of toluene and low boiling point substances are removed by distillation to obtain a light yellow liquid dodecyl dimethoxy (phosphorheterocyclic methoxy) silane. The yield of the product is 97.6%. Its flash point (open cup):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com