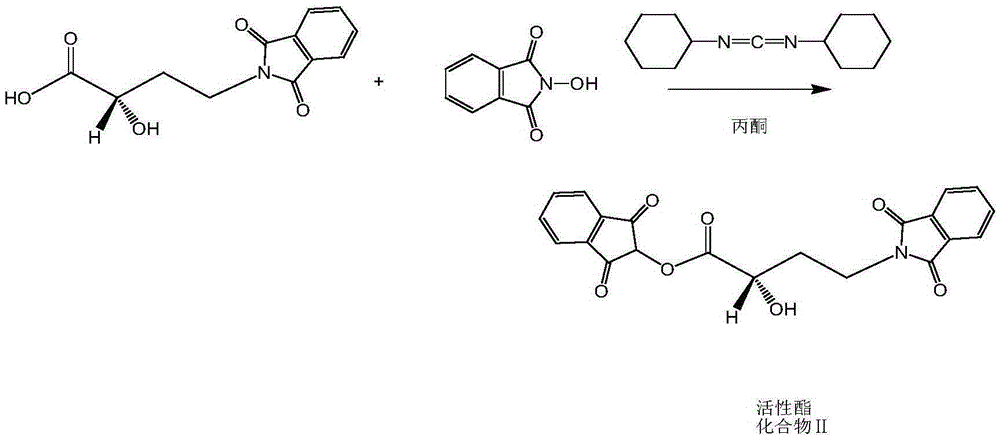
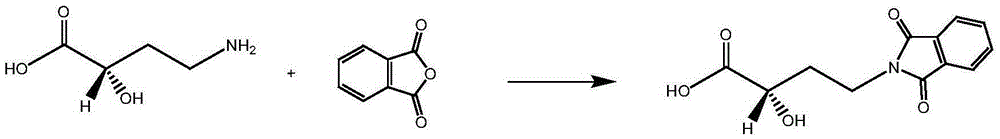Synthetic method of environment-friendly amikacin
A technology of amikacin and a synthesis method, applied in the field of medicine, can solve the problems of high environmental harm, environmental harm, poor purity, etc., and achieve the effects of shortening the synthesis route, reducing waste water and solid waste, and reducing the harm to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Weigh 16.2g (0.11mol) of compound A (2(S)-2-hydroxy-4-carbamoylbutyric acid), 26.7g of N-hydroxyphthalimide, add 500ml of acetone, and heat up Dissolve; lower the temperature to 30°C, add 45.3g DCC, react for 60 minutes; filter to remove the white precipitate; rinse the filter cake twice with 50ml acetone, and combine the filtrate to obtain an active ester / acetone solution for use;
[0049] (2) Weigh 127.8g (0.1mol) of kanamycin A silanide (calculated as full protection), dissolve it with 500ml of acetone, add the above active ester, and react at 5°C for 2 hours to obtain an acylated product;
[0050] (3) Add concentrated hydrochloric acid to the above acylated product to adjust the pH to 2.34, stir for 1 hour, distill under reduced pressure to remove the solvent, add a small amount of water to the above material liquid, and control the material liquid concentration to 82.6g / L;
[0051] (4) Cool down to -10°C, add 60ml of 30% liquid caustic soda, and control the temperatur...
Embodiment 2
[0054] (1) Weigh 16.2g (0.11mol) of compound A (2(S)-2-hydroxy-4-carbamoylbutyric acid), 26.5g of N-hydroxyphthalimide, add 500ml of acetone, and heat up Dissolve; cool to 30°C, add 45.0g DCC, react for 65 minutes; filter to remove the white precipitate; rinse the filter cake twice with 50ml of acetone, combine the filtrate to obtain an active ester / acetone solution for use;
[0055] (2) Weigh 127.8g (0.1mol) of kanamycin A silanide (calculated as full protection), dissolve it with 500ml of acetone, add the above active ester, and react at 5°C for 2 hours to obtain an acylated product;
[0056] (3) Add concentrated hydrochloric acid to the above acylated product to adjust the pH=2.54, stir for 1 hour, distill under reduced pressure to remove the solvent, add a small amount of water to the above material liquid, and control the material liquid concentration to 82.0g / L;
[0057] (4) Cool down to -15°C, add 58ml of 30% liquid caustic soda, control the temperature of the material liquid ...
Embodiment 3
[0060] (1) Weigh 16.2g (0.11mol) of compound A (2(S)-2-hydroxy-4-carbamoylbutyric acid), 27.0g of N-hydroxyphthalimide, add 500ml of acetone, and heat up Dissolve; Cool down to 30°C, add 45.5g DCC, react for 55 minutes; filter to remove the white precipitate; rinse the filter cake twice with 50ml acetone, combine the filtrate to obtain the active ester / acetone solution for use;
[0061] (2) Weigh 127.8g (0.1mol) of kanamycin A silanide (calculated as full protection), dissolve it with 500ml of acetone, add the above active ester, and react at 5°C for 2 hours to obtain an acylated product;
[0062] (3) Add concentrated hydrochloric acid to the above acylated product to adjust the pH=2.76, stir for 1 hour, distill under reduced pressure to remove the solvent, add a small amount of water to the above feed liquid, and control the feed liquid concentration to 80.6g / L;
[0063] (4) Cool down to -10°C, add 62ml of 30% liquid caustic soda, control the temperature of the material liquid to -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com