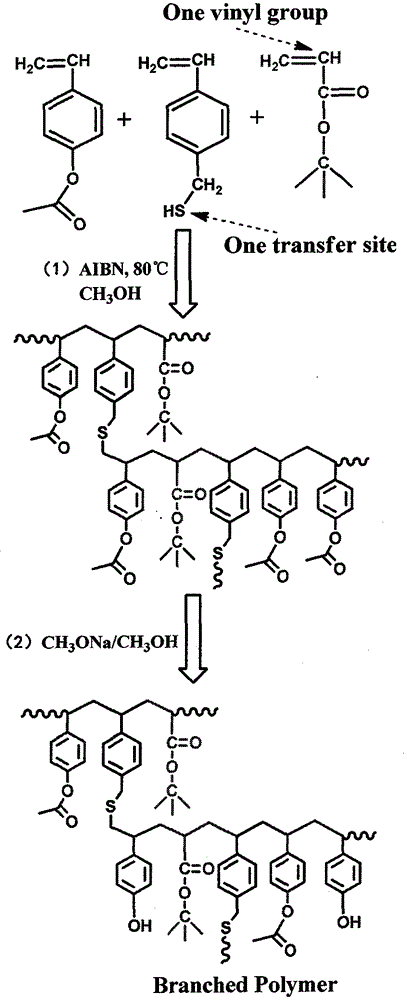
Branched poly (p-hydroxystyrene) copolymer used for 248nm photoresist
A technology of hydroxystyrene and acetoxystyrene, which is applied in the field of branched poly(p-hydroxystyrene) copolymer and its preparation, to achieve the effects of high light transmission, easy preparation and operation, and weak intermolecular entanglement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
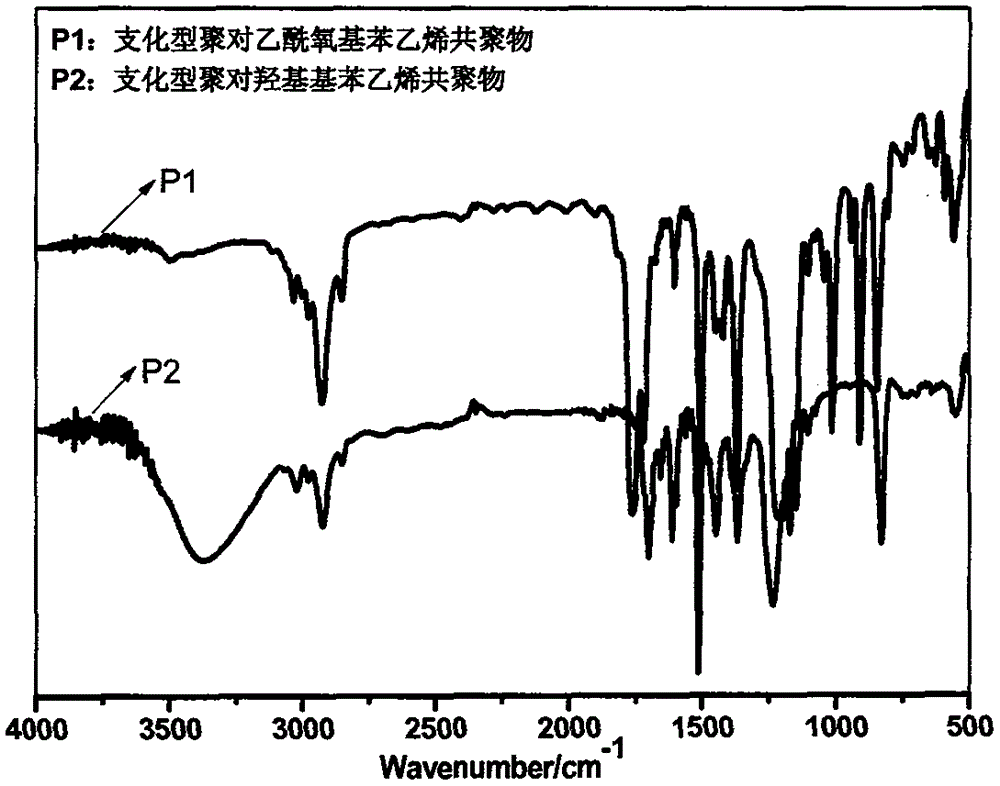
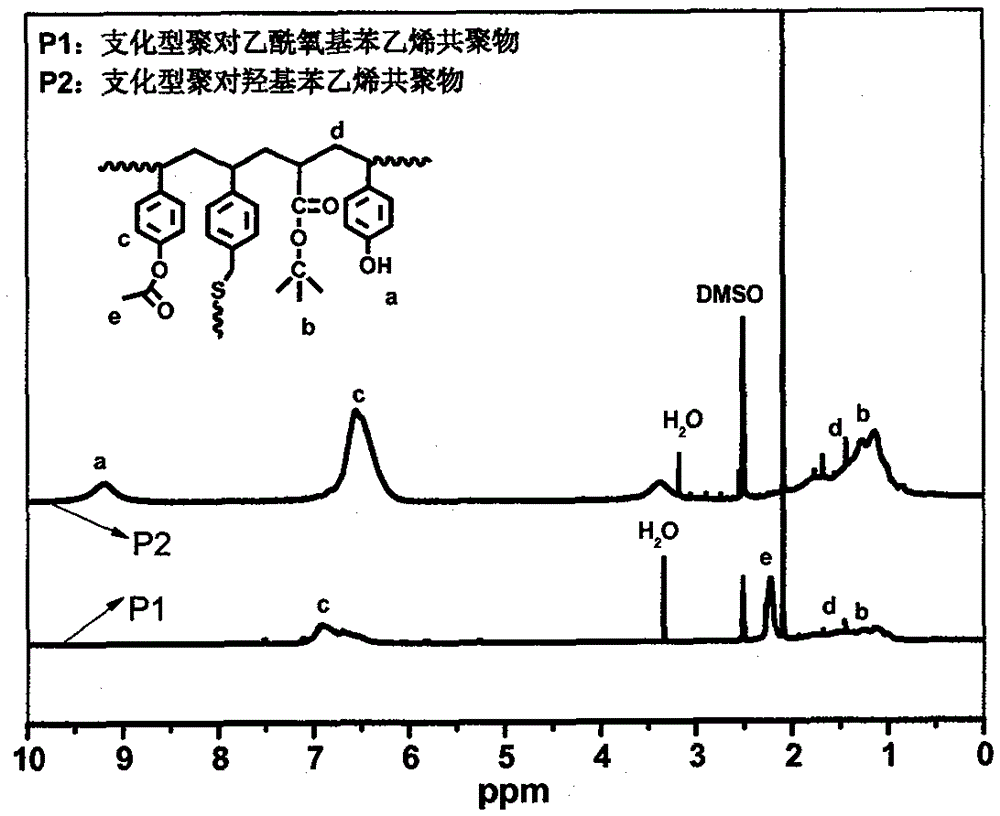
[0030] (1) Synthesis of branched poly(4-acetoxystyrene copolymer): Into a 100ml three-necked round bottom flask equipped with a thermometer, reflux condenser and mechanical stirring, 11.259g (69.42mmol) of 4-acetoxybenzene was added in sequence Ethylene, (12.25mmol) 1.570g tert-butyl acrylate, (1.67mmol) 0.250g 4-vinylbenzyl mercaptan, (2.50mmol) 0.411g AIBN, and 31.476g of anhydrous methanol as polymerization solvent, and place the flask at a constant temperature In an oil bath, heat and stir to 40°C, and after blowing nitrogen for 10 minutes, the system is closed, the temperature is raised to 75°C, and the reaction is stirred at a constant temperature for 24 hours to obtain a branched poly(p-acetoxystyrene) copolymer. Its infrared, nuclear magnetic and ultraviolet spectra are respectively figure 2 , 3 , P1 in 4.
[0031] It can be seen from the infrared: 3000cm -1 Saturated and unsaturated C-H stretching vibration peaks on the left and right, 1770cm -1 Where is the C=O stretch...
Embodiment 2
[0035] (1) Synthesis of branched poly-4-acetoxystyrene copolymer: Into a 100ml three-necked round bottom flask equipped with a thermometer, reflux condenser, and mechanical stirring, 11.259g of 4-acetoxybenzene was sequentially added (68.00mmol) Ethylene, (12.00mmol) 1.570g tert-butyl acrylate, (3.33mmol) 0.250g 4-vinylbenzyl mercaptan, (2.50mmol) 0.411g AIBN, and 31.449g of anhydrous methanol as polymerization solvent, put the flask at a constant temperature In an oil bath, heat and stir to 40°C, and after blowing nitrogen for 10 minutes, the system is closed, the temperature is raised to 75°C, and the reaction is stirred at a constant temperature for 24 hours to obtain a branched poly(p-acetoxystyrene) copolymer.
[0036] (2) Deacetoxylation of branched poly4-acetoxystyrene copolymer: Change the reflux device in (1) to a distillation device, and raise the temperature to 80°C. Add 15g of anhydrous methanol and 1.255g of sodium methoxide to the reaction system of (1), keep the re...
Embodiment 3
[0038] (1) Synthesis of branched poly(4-acetoxystyrene copolymer): Into a 100ml three-necked round bottom flask equipped with a thermometer, reflux condenser and mechanical stirring, 11.259g (66.58mmol) of 4-acetoxybenzene was sequentially added Ethylene, (11.75mmol) 1.570g tert-butyl acrylate, (5.00mmol) 0.250g 4-vinylbenzyl mercaptan, (2.50mmol) 0.411g AIBN, and 31.423g of anhydrous methanol as polymerization solvent, and place the flask at a constant temperature In an oil bath, heat and stir to 40°C, and after blowing nitrogen for 10 minutes, the system is closed, the temperature is raised to 75°C, and the reaction is stirred at a constant temperature for 24 hours to obtain a branched poly(p-acetoxystyrene) copolymer.
[0039] (2) Deacetoxylation of branched poly4-acetoxystyrene copolymer: Change the reflux device in (1) to a distillation device, and raise the temperature to 80°C. Add 15g of anhydrous methanol and 1.228g of sodium methoxide to the reaction system of (1), keep ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com