Kit and method for detecting duck hepatitis A viruses through dual TaqMan fluorescent quantitation RT-PCR
A duck hepatitis A virus, fluorescence quantitative technology, applied in microorganism-based methods, biochemical equipment and methods, microorganism determination/inspection and other directions, can solve the problems of no economic loss, difficult to standardize, low sensitivity, etc. Simple, high sensitivity, good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Design and synthesis of embodiment 1 primer and TaqMan probe
[0030] According to the gene sequences of DHAV-1 (accession number: JQ301467.1), DHAV-2 (accession number: EF067924.1; EF067923.1), DHAV-3 (accession number EU877916.1) in GenBank, by comparison , select the conserved, specific base sequence (nucleotide sequence as shown in SEQIDNO.1, SEQIDNO.2) in the VP0 region of DHAV-1 and the VP3 region of DHAV-3, adopt PrimerPrimer5.0 software to carry out specific primer and For the design of TaqMan probes, see Tables 1 and 2 for details.
[0031] Table 1 Standard primers
[0032]
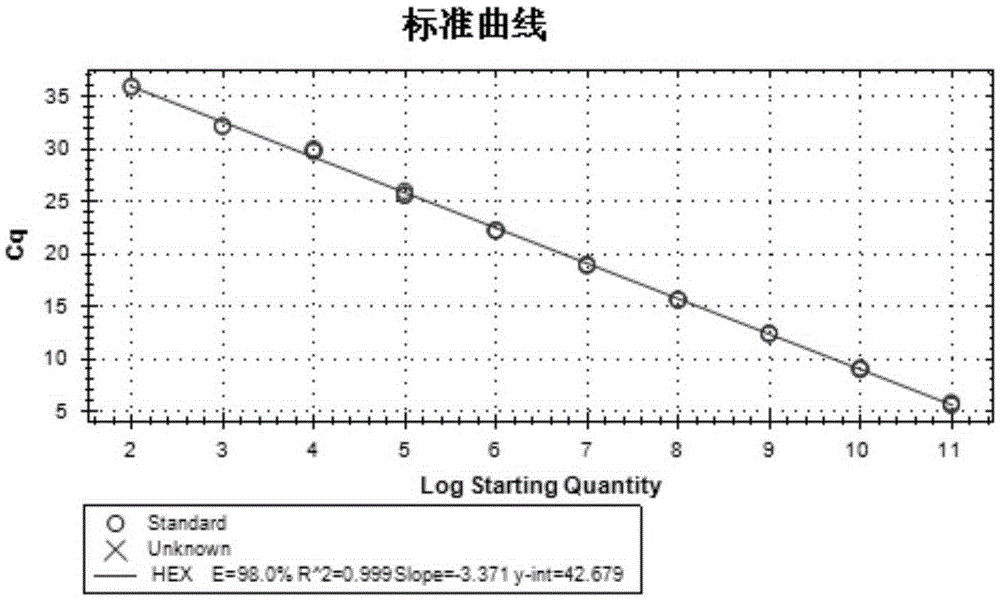
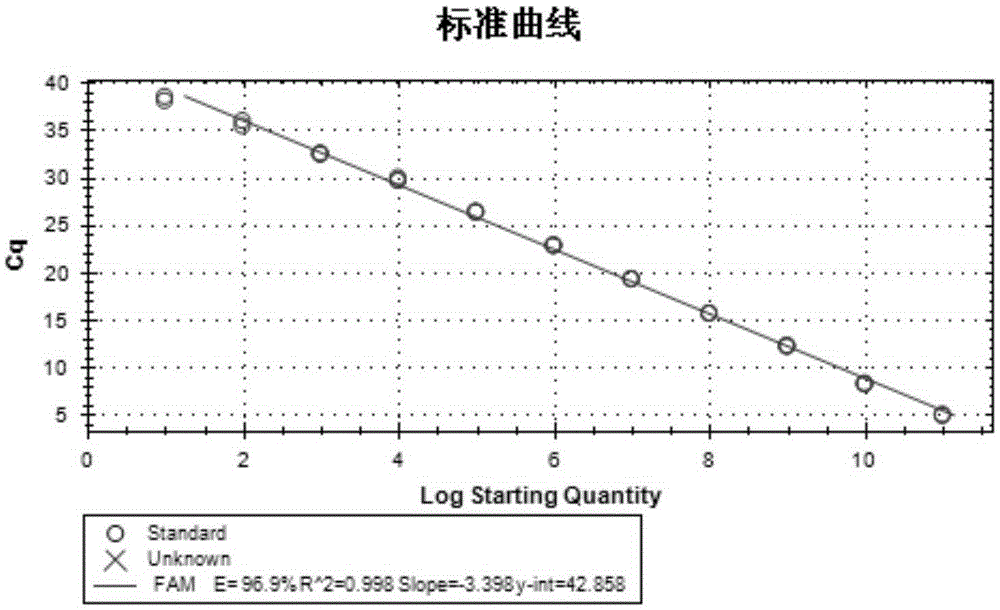
[0033] Table 2 Quantitative primers and probes
[0034]
[0035] See SEQ ID NO.15 for the expected sequence of PCR amplification of primer pair 1, see SEQ ID NO.16 for the expected sequence of PCR amplification of primer pair 2; see SEQ ID NO.13 for the expected sequence of the quantitative PCR amplification fragment (VP0) of primer pair 3, and see SEQ ID NO. The expected sequence...
Embodiment 2
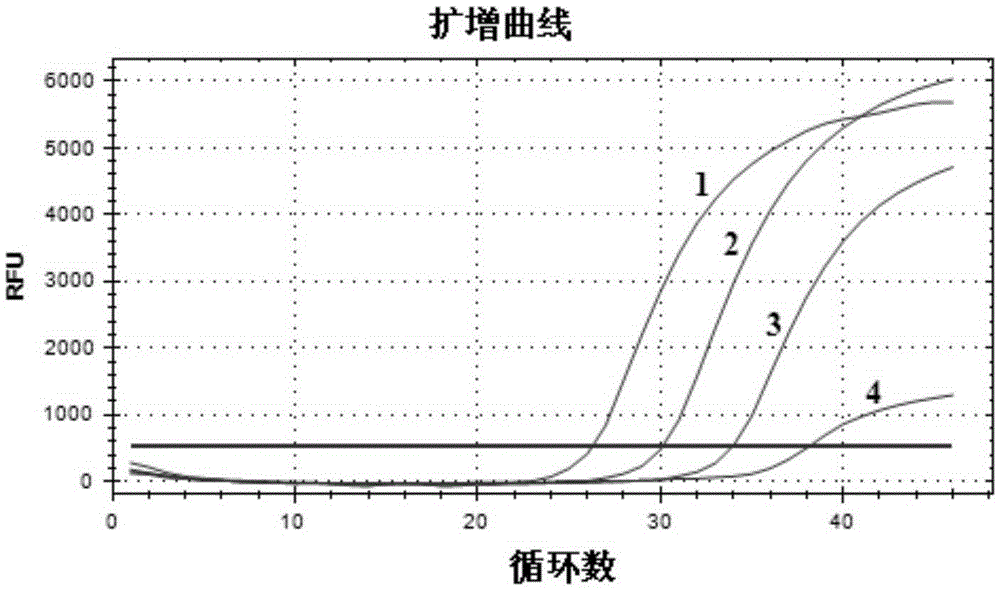
[0036] Embodiment 2 fluorescence quantitative RT-PCR detects
[0037] 1. Determination of fluorescent quantitative RT-PCR detection method
[0038] 1.1 Sample preparation
[0039] 1.1.1 RNA nucleic acid extraction
[0040] ①Take 0.5mL of allantoic fluid sample into a 1.5mL centrifuge tube treated with DEPC;
[0041] ②Add 0.5mL Trizol, shake and mix well, and let stand at room temperature for 10min;
[0042] ③Add 0.2mL chloroform, vibrate vigorously to mix well, fully emulsify until there is no phase separation, and place at room temperature for 5 minutes;
[0043] ④ Centrifuge at 12000rpm for 15min at 4°C;
[0044] ⑤ Take out the centrifuge tube carefully without shaking, and carefully pipette the supernatant into another 1.5mL centrifuge tube treated with DEPC;
[0045] ⑥ Add an equal volume of isopropanol to the supernatant, invert the centrifuge tube up and down to mix, and let stand at 4°C for 5-10 minutes to precipitate nucleic acids;
[0046] ⑦ Centrifuge at 12000r...
Embodiment 3
[0093] The assembly of embodiment 3 detection kits
[0094] According to the research results of Example 1 and Example 2, a detection kit was assembled for use, and the composition of the kit is shown in Table 10.
[0095] Table 10 Kit Composition
[0096]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com