A kind of method utilizing anhydrous lithium hydroxide to prepare high-purity lithium oxide
A technology of anhydrous lithium hydroxide and lithium oxide, applied in the chemical field, can solve problems such as potential safety hazards, environmental pollution, and increased production costs, and achieve the effects of low production costs, reduced environmental pollution, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
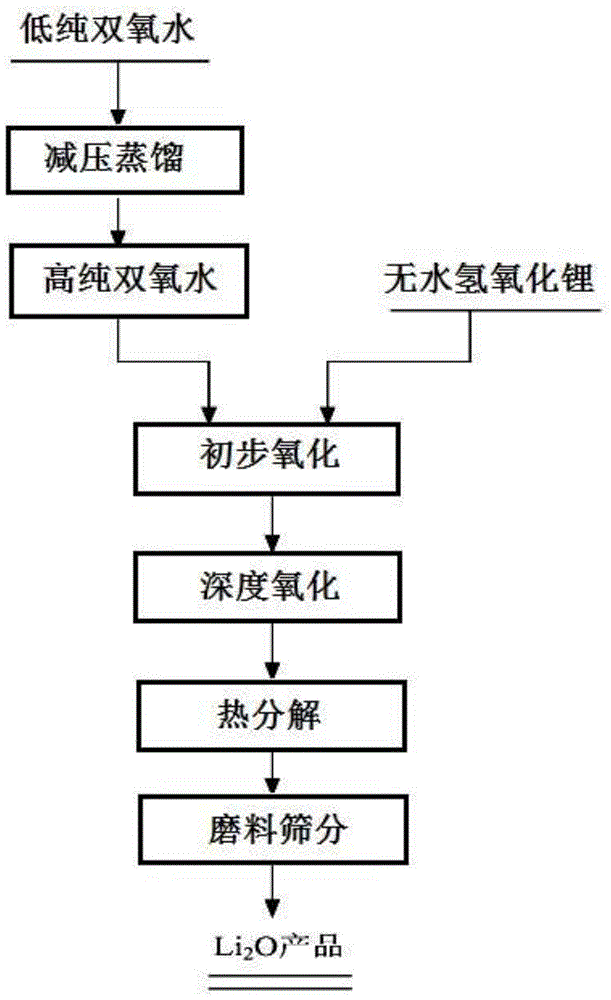
[0032] 1) Distillation under reduced pressure: 5.0kg of hydrogen peroxide with a concentration of 50% was added to the airtight reaction vessel A, and under a relative vacuum of -0.01MPa, the hydrogen peroxide was distilled under reduced pressure for 2 hours, and the distillation temperature was 40°C to obtain a concentration of 95% hydrogen peroxide. concentrated hydrogen peroxide;
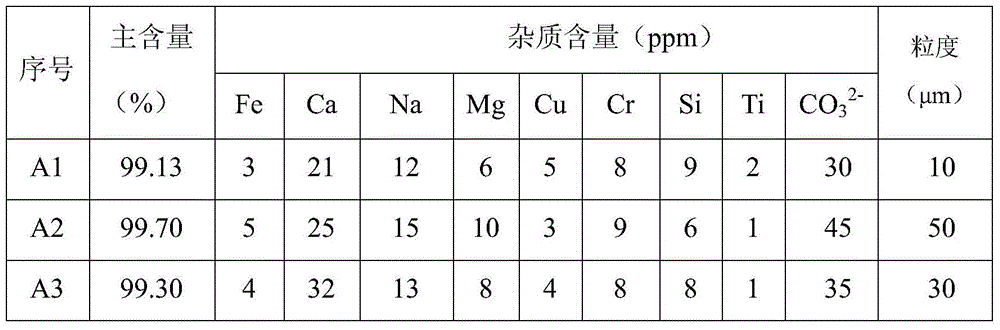
[0033] 2) Preliminary oxidation: Press 2.5kg of high-concentration hydrogen peroxide obtained in step 1) into another closed reaction vessel B containing 3.4kg of anhydrous lithium hydroxide, then vacuumize to -0.01MPa, and adjust the temperature to 40°C , stirred for 1h, the purity of lithium hydroxide is 99.95%, particle size D 50 5 μm;
[0034] 3) Deep oxidation: raise the temperature of the material in the reaction vessel B to 60°C, keep the vacuum degree in the vessel at -0.01MPa, and heat for 1 hour to obtain lithium peroxide;
[0035] 4) Thermal decomposition: heat up the lithium peroxid...
Embodiment 2
[0038] 1) Distillation under reduced pressure: Add 8.0 kg of hydrogen peroxide with a concentration of 70% into the airtight reaction vessel A, and distill under reduced pressure for 4 hours at a relative vacuum of -0.09 MPa at a distillation temperature of 50°C to obtain hydrogen peroxide with a concentration of 97%. ;
[0039] 2) Preliminary oxidation: Press 5.5kg of high-concentration hydrogen peroxide obtained in step 1) into another closed reaction vessel B containing 7.0kg of anhydrous lithium hydroxide, then vacuumize to -0.09MPa, and adjust the temperature to 50°C , stirred and reacted for 5h, the purity of lithium hydroxide was 99.93%, and the particle size was D 50 20μm;
[0040] 3) Deep oxidation: raise the temperature of the material in the reaction vessel B to 100°C, keep the vacuum degree in the vessel at -0.09MPa, and heat for 5 hours to obtain lithium peroxide;
[0041] 4) Thermal decomposition: heat up the lithium peroxide obtained in step 3) to 500° C., keep ...
Embodiment 3
[0044] 1) Distillation under reduced pressure: Add 20kg of hydrogen peroxide with a concentration of 30% into the airtight reaction vessel A, and distill under reduced pressure for 2.5 hours at a relative vacuum of -0.05MPa, the distillation temperature is 45°C, and the concentration is 96%. hydrogen peroxide;
[0045] 2) Preliminary oxidation: Press 6.0kg of high-concentration hydrogen peroxide obtained in step 1) into another closed reaction vessel B containing 7.8kg of anhydrous lithium hydroxide, then vacuumize to -0.05MPa, and adjust the temperature to 45°C , stirred for 3h, the purity of lithium hydroxide is 99.95%, particle size D 50 13μm;
[0046] 3) Deep oxidation: raise the temperature of the material in the reaction vessel B to 80°C, keep the vacuum degree in the vessel at -0.05MPa, and heat for 3 hours to obtain lithium peroxide;
[0047] 4) Thermal decomposition: heat up the lithium peroxide obtained in step 3) to 450° C., keep the relative vacuum of the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com