A kind of preparation method of cephalexin raw material and capsule
A technology of cephalexin and raw materials, applied in the field of medicine, can solve the problems of affecting the yield, difficulty in enzyme separation, and low production efficiency, and achieve the effects of increasing production yield, overcoming difficulties in enzyme separation, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of cephalexin raw material, comprising the following steps:
[0026] (1) Add 120ml of deionized water and 20g of 7-ADCA in the reaction flask, stir to dissolve, and dissolve L-phenylglycine methyl ester hydrochloride (18.0g in terms of L-phenylglycine methyl ester) with 50ml of deionized water. The solution was slowly added to the 7-ADCA aqueous solution within 45 minutes, then 15 g of immobilized penicillin acylase (with an activity of 23 U / g) was added, and the acylation reaction was carried out at 15 ° C. The reaction time was 3.0 hours, and ammonia water was used during the reaction to maintain The pH is at 6.2.
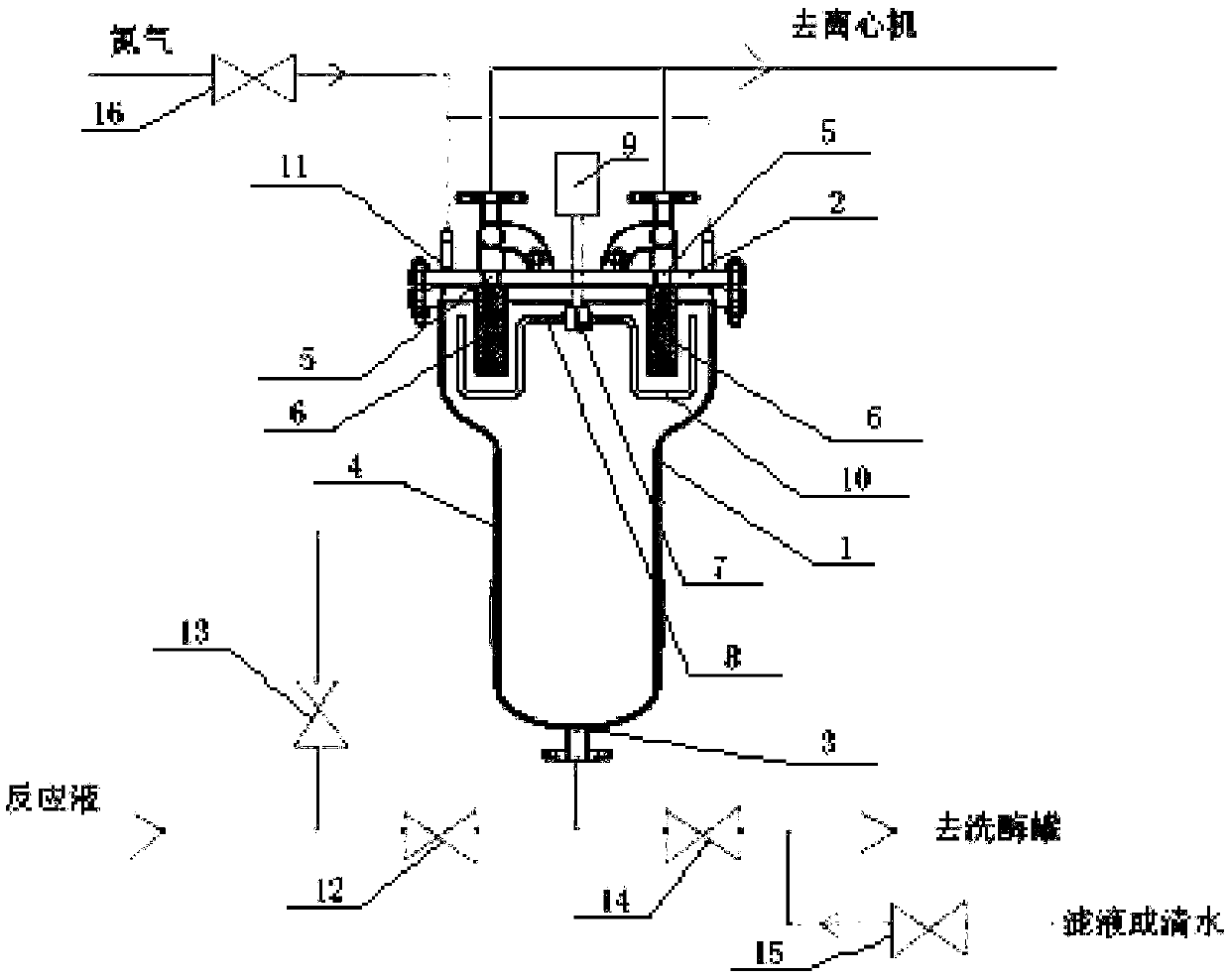
[0027] (2) After the reaction is terminated, adjust the viscosity of the reaction solution to 50mPa·s with deionized water, and then use the penicillin acylase separator (see figure 1 ) for initial separation, the penicillin acylase separator includes a tank 1 and a cover 2 sealed thereto, the bottom of the tank 1 is provided with a b...
Embodiment 2
[0032] A preparation method of cephalexin raw material, comprising the following steps:
[0033] (1) Add 140ml of deionized water and 20g of 7-ADCA into the reaction flask, stir to dissolve, dissolve the L-phenylglycine methyl ester sulfate (18.5g in terms of L-phenylglycine methyl ester) with 50ml of deionized water, and dissolve the solution Slowly add to 7-ADCA aqueous solution within 60min, then add 18g of immobilized penicillin acylase (activity is 23U / g), carry out acylation reaction at 18°C, the reaction time is 2.8 hours, maintain the pH with ammonia water during the reaction In 6.6.
[0034] (2) After the reaction is terminated, adjust the viscosity of the reaction solution to 100mPa·s with deionized water, and then use the penicillin acylase separator (see figure 1 ) for initial separation, the penicillin acylase separator includes a tank 1 and a cover 2 sealed thereto, the bottom of the tank 1 is provided with a bottom inlet and outlet 3 and the middle of the tank ...
Embodiment 3
[0039] A preparation method of cephalexin raw material, comprising the following steps:
[0040] (1) Add 180ml of deionized water and 20g of 7-ADCA into the reaction bottle, stir to dissolve, slowly add 21.6g of L-phenylglycine methyl ester into the 7-ADCA aqueous solution within 75min, and then add 19g of immobilized penicillin acylase (activity is 23U / g), acylation reaction is carried out at 20°C, the reaction time is 2.5 hours, and the pH is maintained at 7.0 with ammonia water during the reaction;
[0041] (2) After the reaction is terminated, adjust the viscosity of the reaction solution to 200mPa·s with deionized water, and then use the penicillin acylase separator (see figure 1 ) for initial separation, the penicillin acylase separator includes a tank 1 and a cover 2 sealed thereto, the bottom of the tank 1 is provided with a bottom inlet and outlet 3 and the middle of the tank 1 is provided with an intermediate inlet 4 , the cover 2 is provided with 6 symmetrically ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com