A method for the total synthesis of petrosiol E
A technology of total synthesis and intermediates, applied in the fields of organic chemistry, bulk chemical production, hydrolysis preparation, etc., can solve the problems of difficulty in obtaining Petrosiol-like natural products, difficult to meet the needs of chemical properties and biological activity research, etc. Prospects for industrial production, easy operation, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The following provides an embodiment of the present invention:
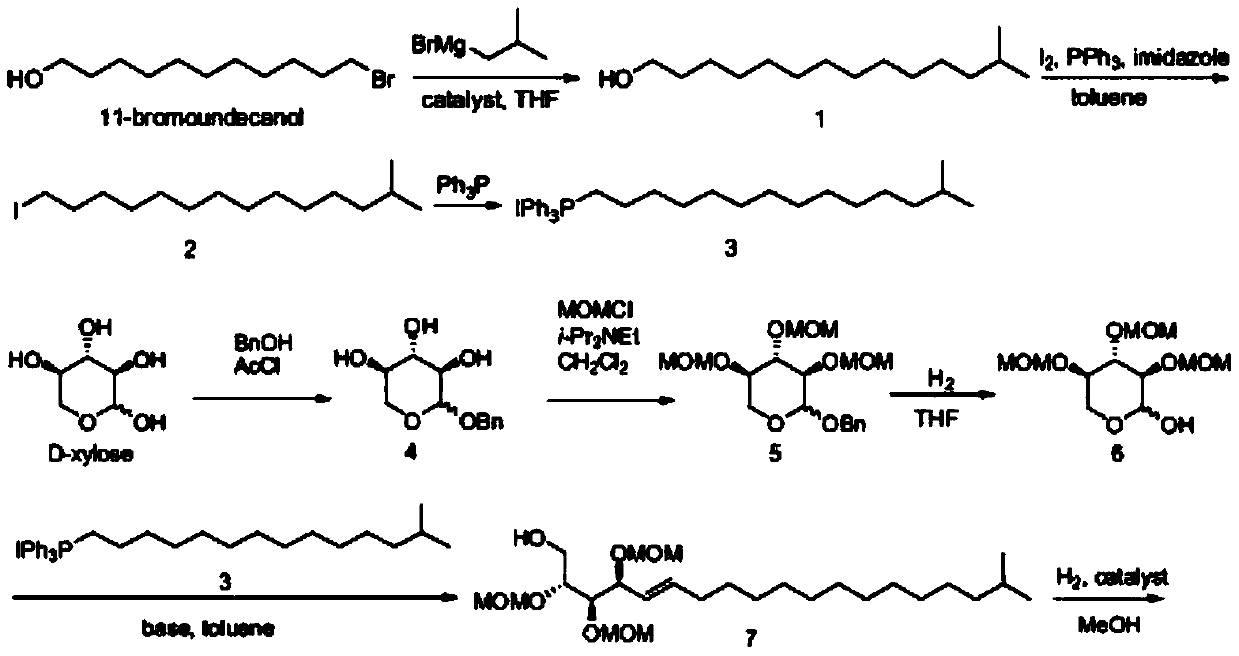
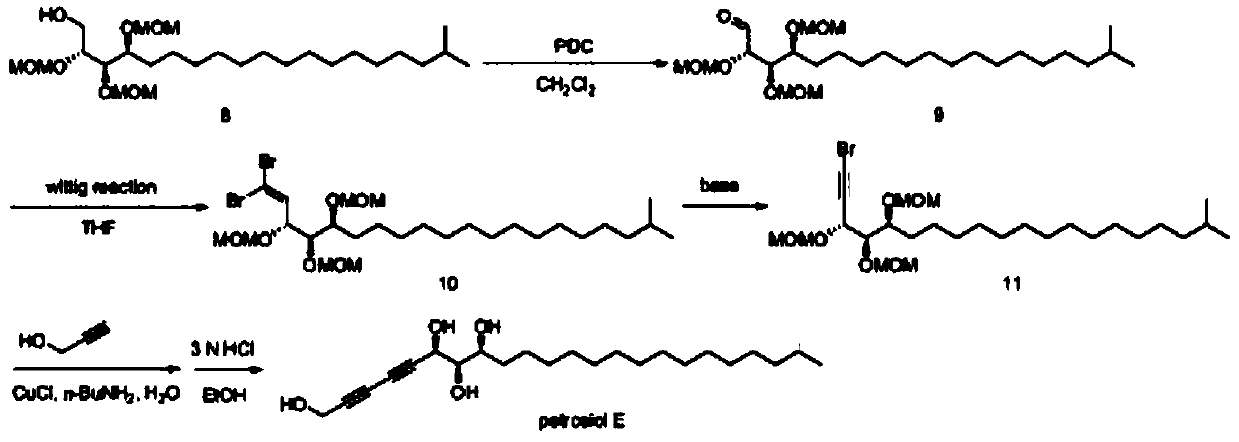
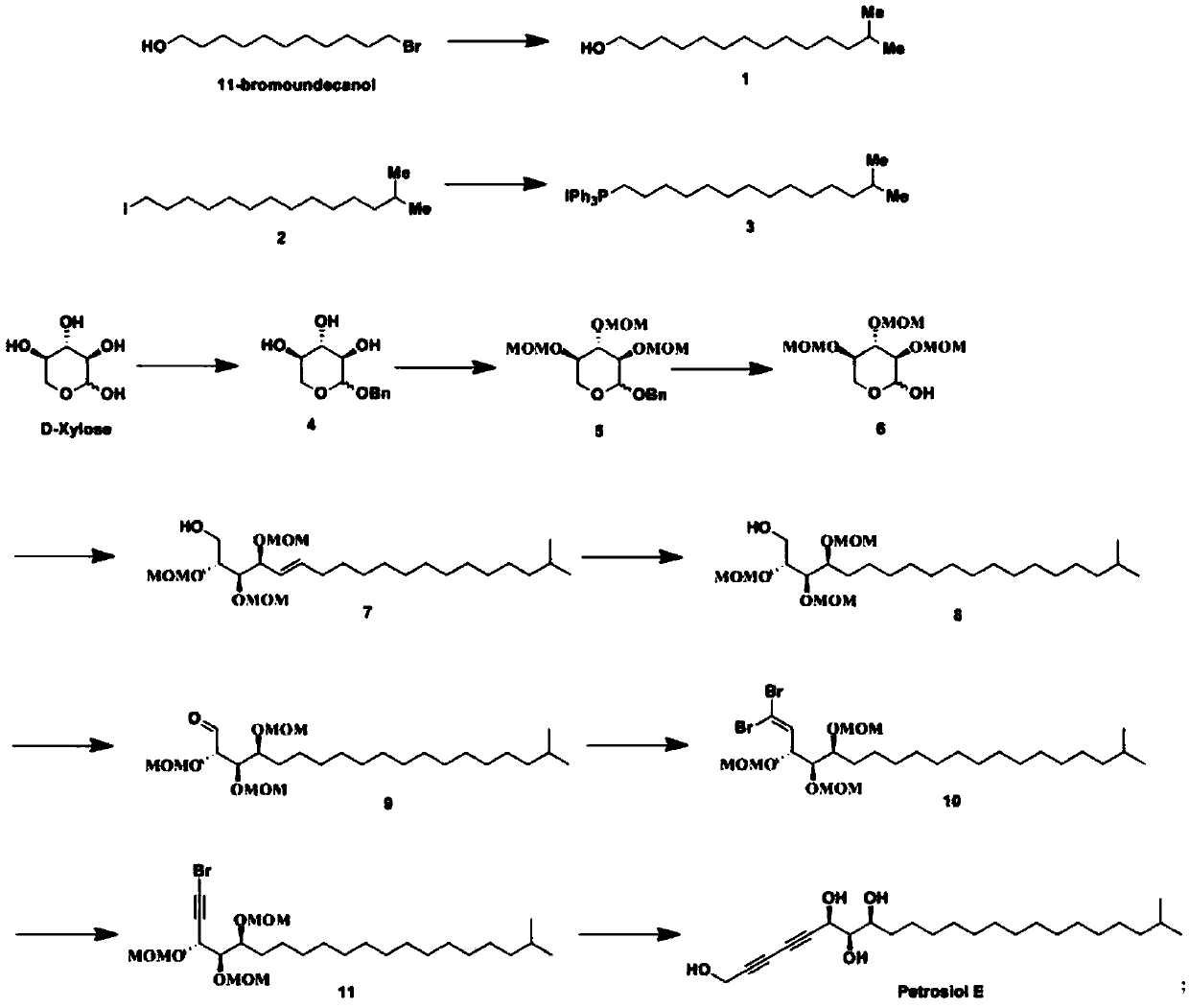
[0022] Synthesis of compound 1:
[0023] Under the condition of -78℃, the tetrahydrofuran solution of isobutylmagnesium bromide (1mol / L, 3.7eqv) and the tetrahydrofuran solution of cuprous iodide (0.1mol / L, 0.03eqv) were added dropwise to the 11-bromo ten One alcohol (1eqv) in tetrahydrofuran solution, react overnight at room temperature, add saturated ammonium chloride solution to quench the reaction, extract with ethyl acetate, wash the organic phase with water, wash with saturated sodium bicarbonate solution, wash with brine, dry, and evaporate to dryness. The crude product of body 1.
[0024] Synthesis of compound 2:
[0025] Intermediate 1 (1eqv), triphenylphosphine (1.5eqv), and imidazole (1.5eqv) were dissolved in toluene, stirred vigorously, iodine (3eqv) was added at 0°C, and the reaction was heated for 2 hours. Wash with saturated sodium thiosulfate solution, extract with ether, wash the organic phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com