Basic fibroblast growth factor (bFGF) specifically bound with chitin as well as coding gene, preparation method and application thereof
A fibroblast, specific binding technology, applied in the field of genetic engineering, can solve the problems of unfavorable blood vessel formation, tissue regeneration, carcinogenic risk, etc., and achieve the effect of promoting proliferation, avoiding excessive release, and good slow release effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1CBD-bFGF
[0049] The gene containing CBD-bFGF is cloned into PET21b expression vector, and the enzyme cutting sites are: NDEI and XholI.
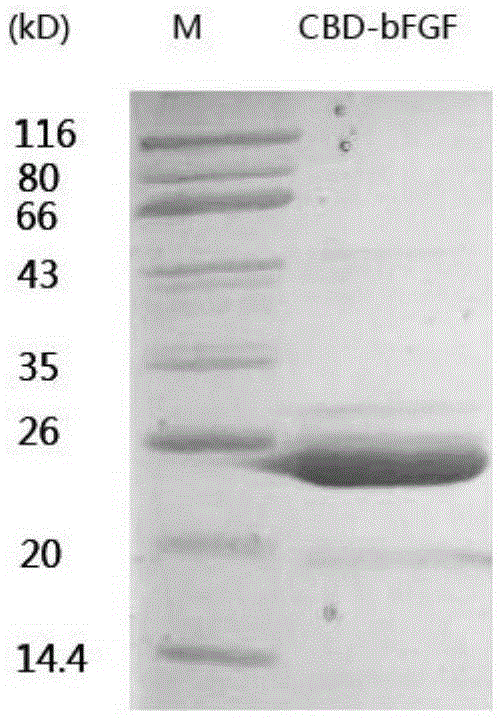
[0050] This vector was transformed into E.coliBL21(DE3), and 0.2mMIPTG was induced to express CBD-bFGF protein at 37℃ for 12h. CBD-bFGF protein was obtained through His-tag packing affinity chromatography. As shown in the figure below: the electrophoresis figure shows that we have purified CBD-bFGF protein, the theoretical molecular weight is 23619.82 and the molecular weight of CBD-bFGF in the electrophoresis figure is basically the same, as figure 1 shown.
Embodiment 2
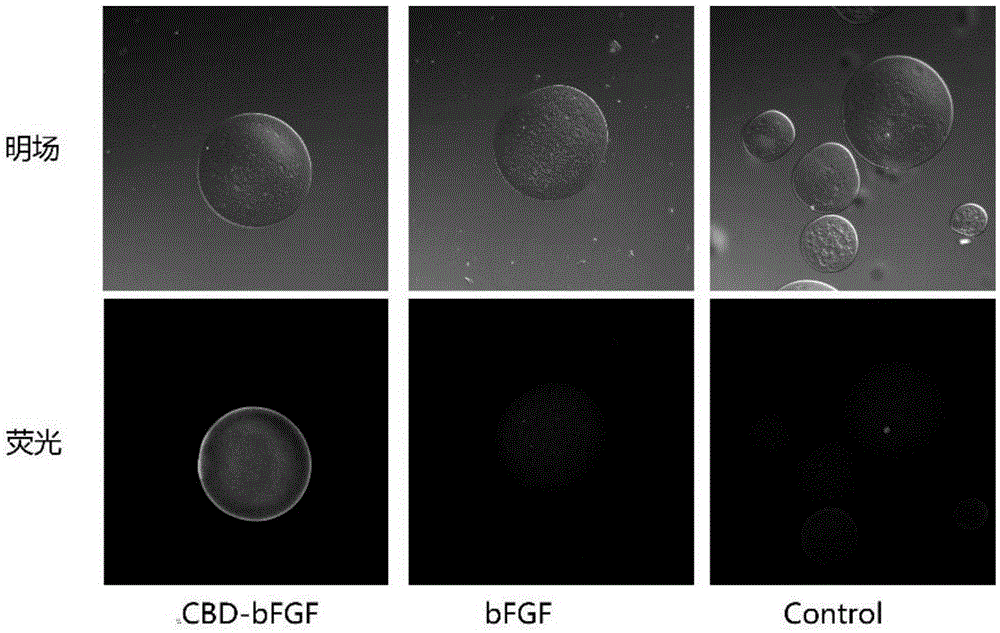
[0051] Example 2 Detection of CBD-bFGF and chitin sphere binding ability
[0052] CBD-bFGF and bFGF are used as detection objects, and PBS is used as control. The method is:
[0053] 1) Add chitin spheres to solutions containing CBD-bFGF (1 μg / mL) and bFGF (1 μg / mL) respectively, and incubate at 37°C for 2 hours;
[0054] 2) Wash the chitin spheres three times with PBS, 5 minutes each time;
[0055] 3) Add the chitin spheres bound with CBD-bFGF and bFGF to the solution containing 10% BSA respectively for sealing;
[0056] 4) Add the chitin spheres bound with CBD-bFGF and bFGF to the antibody containing mouse anti-bFGF respectively, and incubate overnight at 4°C;
[0057] 5), wash the chitin spheres with PBS three times, 5 minutes each time;
[0058] 6) Add the chitin spheres bound with CBD-bFGF and bFGF to the rabbit anti-mouse FITC-labeled antibody, and incubate at 37°C for 2 hours;
[0059] 7), wash the chitin spheres with PBS three times, 5 minutes each time;
[0060]...
Embodiment 3
[0065] The sustained release effect of embodiment 3CBD-bFGF
[0066] To detect the slow-release effect of CBD-bFGF, with bGFG as the control, the method is as follows:
[0067] The chitin membrane is prepared into a disc with a diameter of 8mm;
[0068] bFGF, CBD-bFGF cytokines are prepared into a 20ug / ml solution with PBS;
[0069] Soak the chitin membrane in bFGF and CBD-bFGF solution respectively, overnight at 4°C;
[0070] Take the membranes out of bFGF and CBD-bFGF solutions respectively, and place them in PBS solution;
[0071] Sampling at 1, 3, 5, 8, 11, 14, 17, 20, 24, 48 hour time points;
[0072] According to the method of bFGFElisa kit instructions, detect the content of bFGF in samples at different time points;
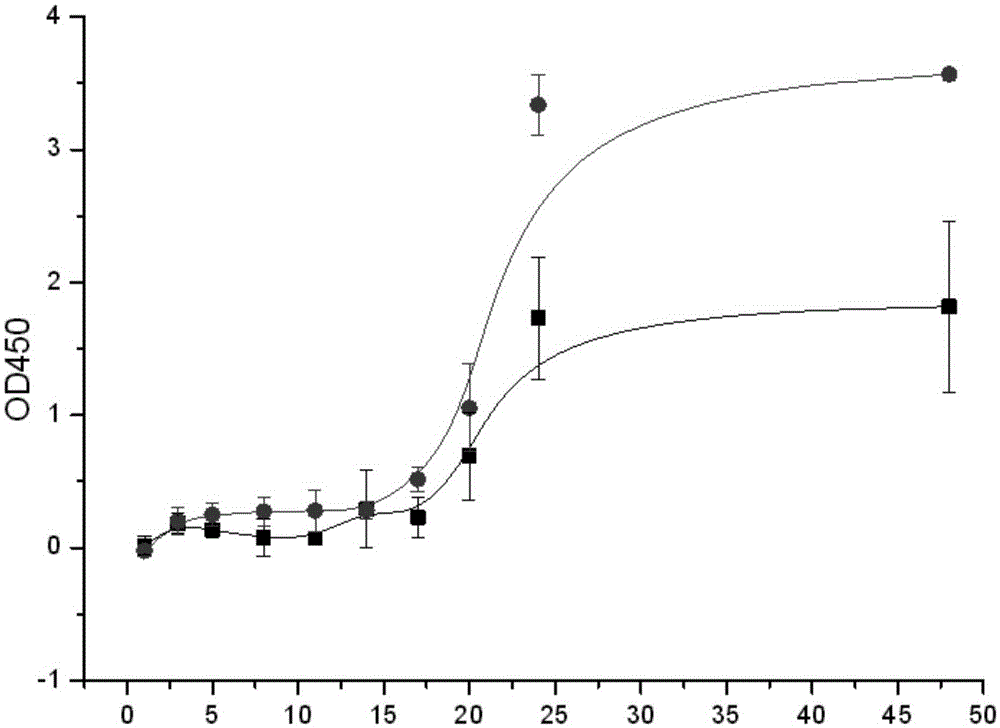
[0073] Use Origin to map, such as image 3 .
[0074] Depend on image 3 It can be seen that from 0 to 48 hours, the cytokine CBD-bFGF with chitin-binding domain has a better slow release ability than native bFGF.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com