Liquid crystalline compound having 2,6-difluorophenyl ether structure and liquid crystal composition thereof
一种化合物、-CH2-的技术,应用在有机化学、液晶材料、有机化学方法等方向,能够解决粘度高、T→i降低等问题,达到低粘度、高混合性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
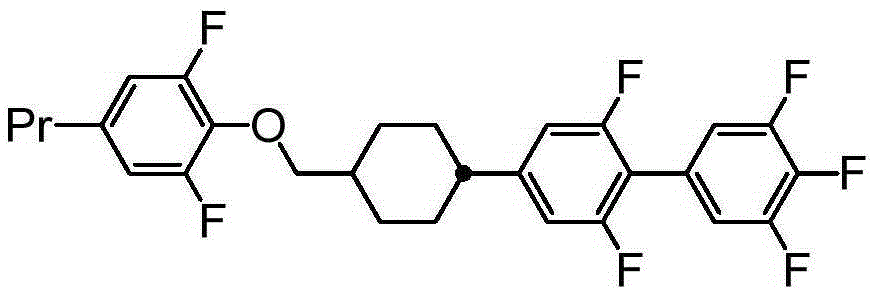
[0292] (Example 1) trans-2-(3,4,5-trifluorophenyl)-5-[(2,6-difluoro-4-propylphenyloxy)methyl]-1,3 -two Production of alkane(1-1)
[0293][chem 47]
[0294]
[0295] (1-1) Under a nitrogen atmosphere, 3,4,5-trifluorobenzaldehyde (7.5 g), 2-hydroxymethyl-1,3-propanediol (5.0 g) and p-toluenesulfonic acid monohydrate ( 0.5 g) was suspended in toluene (30 mL), refluxed, and stirred for 3 hours while distilling off generated water. After standing to cool, saturated aqueous sodium bicarbonate solution (15 mL) was added for liquid separation, the organic layer was washed with saturated aqueous sodium bicarbonate solution and saturated brine, and dried by adding anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure to obtain crude 5-hydroxymethyl-2-(3,4,5-trifluorophenyl)-1,3-bis Alkane (11.3 g).
[0296] (1-2) Under a nitrogen atmosphere, the 5-hydroxymethyl-2-(3,4,5-trifluorophenyl)-1,3-bis Alkane (11.3 g), 2,6-difluoro-4-propylphenol (...
Embodiment 2
[0300] (Example 2) trans-2-[4-(3,4,5-trifluorophenyl)phenyl]-5-[(2,6-difluoro-4-propylphenyloxy)methyl base]-1,3-di Manufacturing of alkanes
[0301] [chem 48]
[0302]
[0303] (2-1) Under a nitrogen atmosphere, mix 4-bromobenzaldehyde (20.0g), tetrakis(triphenylphosphine)palladium(0) (2.5g), THF (100mL) and 2mol% potassium carbonate aqueous solution (110mL) Mix and heat to 60°C. A solution in which 3,4,5-trifluorophenylboronic acid (20.9 g) was dissolved in THF (60 mL) was slowly added under heating, and stirred at 60° C. for 15 hours. After standing to cool, the insoluble matter was filtered, toluene (30 mL) was added for liquid separation, the organic layer was washed twice with saturated brine (100 mL), and dried with anhydrous sodium sulfate. After distilling off the solvent under reduced pressure, it purified by silica gel column chromatography and recrystallized from a mixed solvent of toluene and hexane to obtain 4-(3,4,5-trifluorophenyl)benzaldehyde (24.6 g)....
Embodiment 3
[0308] (Example 3) trans-2-[4-(3,4,5-trifluorophenyl)-3-fluorophenyl]-5-[(2,6-difluoro-4-propylphenyl Oxy)methyl]-1,3-bis Manufacturing of alkanes
[0309] [chem 49]
[0310]
[0311] (3-1) Under a nitrogen atmosphere, 3-fluorobenzaldehyde (6.0 g), 2-hydroxymethyl-1,3-propanediol (5.0 g) and p-toluenesulfonic acid monohydrate (0.5 g) were suspended in Toluene (30 mL) was refluxed, and stirred for 3 hours while distilling off generated water. After standing to cool, saturated aqueous sodium bicarbonate solution (15 mL) was added for liquid separation, the organic layer was washed with saturated aqueous sodium bicarbonate solution and saturated brine, and dried by adding anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure to obtain crude 5-hydroxymethyl-2-(3-fluorophenyl)-1,3-di Alkane (10.1 g).
[0312] (3-2) Under a nitrogen atmosphere, the 5-hydroxymethyl-2-(3-fluorophenyl)-1,3-bis obtained from (3-1) Alkane (10.1g), triphenylp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com