Preparation method for (S)-oxiracetam
A synthesis method and C1-C6 technology, applied in the field of oxiracetam, can solve the problems of affecting the purity and yield of oxiracetam, difficult to recover, easy to be destroyed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
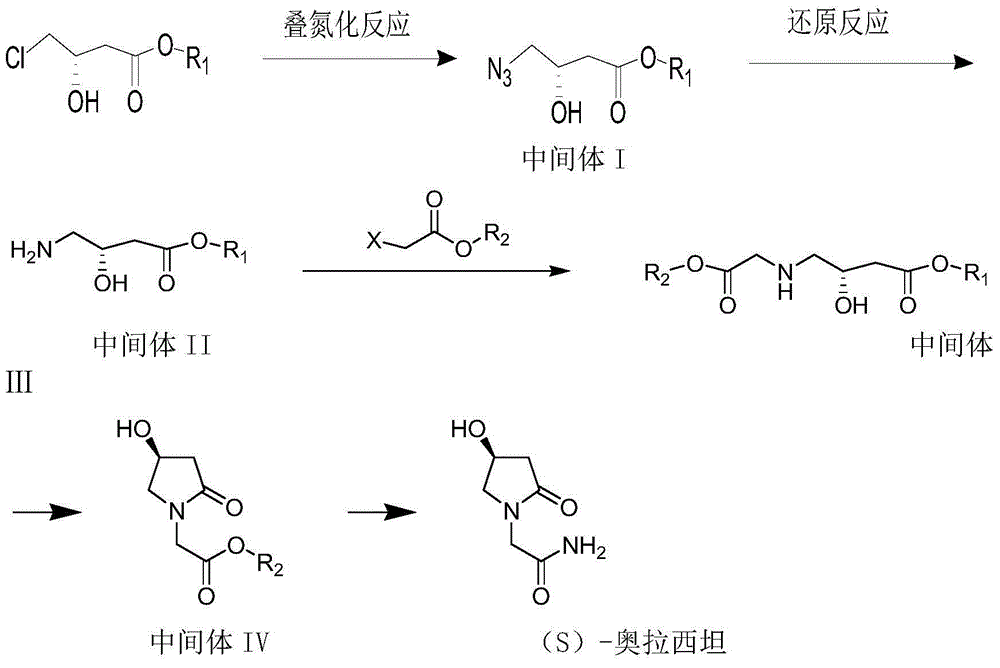
[0069] A kind of synthetic method of (S)-oxiracetam, it carries out as follows,
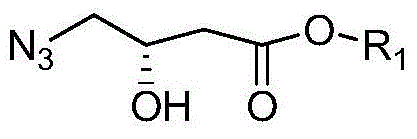
[0070] (1) Preparation of Intermediate I:
[0071] Take 50g of the raw material S-methyl 4-chloro-3-hydroxybutyrate, put it into a single-neck bottle, add 50ml of DMF, stir, cool in an ice-water bath, add 50g of sodium azide, and keep the temperature not exceeding 40°C. The temperature was raised to 60°C. After 2 hours of reaction, the reaction was stopped to obtain a yellow solution. Add 100 ml of water, extract with 100 ml of ethyl acetate, concentrate to remove ethyl acetate, and obtain Intermediate I as a yellow oil. After nuclear magnetic detection, the intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 2H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem, m, 2H) , 3.75(s, 3H), 4.40(m, 1H), 3.70(s, 1H). Intermediate I is: R1 is methyl.
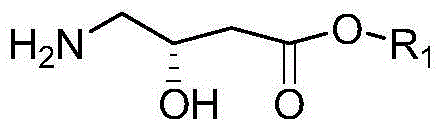
[0072] (2) Preparation of Intermediate II
[0073] The intermediate I obtained in step (2) was dissolved in 500ml of methanol, cooled to an e...
Embodiment 2
[0083] A kind of synthetic method of (S)-oxiracetam, it carries out as follows,
[0084] (1) Preparation of Intermediate I:
[0085] Weigh 50kg of methyl S-4-chloro-3-hydroxybutyrate, add it to a 500L azidation reaction kettle, add DMF50L, stir evenly, cool the jacket in an ice-water bath, add 50Kg of sodium azide, and keep the temperature constant When the temperature exceeds 40°C, the ice water in the jacket is pressed out, and the jacket is passed through hot water to raise the internal temperature to 60°C. After 2 hours of reaction, the reaction was stopped to obtain a yellow solution. Add 100 L of water to the kettle, extract with 100 L of ethyl acetate, separate and discard the water phase, concentrate the organic phase to remove ethyl acetate, and obtain Intermediate I as a yellow oil. After nuclear magnetic detection, the intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 2H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem, m, 2H) , 3.75(s, 3H), 4.40(m, 1H)...
Embodiment 3
[0098] 1, a kind of synthetic method of (S)-oxiracetam, follow the steps:
[0099] (1) Stir S-4-chloro-3-hydroxybutyric acid ethyl ester with 18 times the weight of DMSO and 1 times the weight of sodium azide, and react with azidation at 60°C for about 5 hours. The raw materials are basically The reaction is complete, stop the reaction, directly concentrate to remove the solvent, and solidify at low temperature to obtain intermediate I; meanwhile, the above solvents also use DMF, n-propanol, isopropanol, n-butanol, tert-butanol, toluene or cyclopentanol, etc. Preparation of intermediate I, and finally by nuclear magnetic detection, the prepared intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 5H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem, m, 2H), 4.40 (m, 1H), 3.70 (s, 1H).
[0100] (2) With the intermediate I obtained from step (1), in 15 weight times of ethanol of S-4-chloro-3-hydroxybutyrate ethyl ester, add 0.5 times of weight of 10% platinum carbon catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com