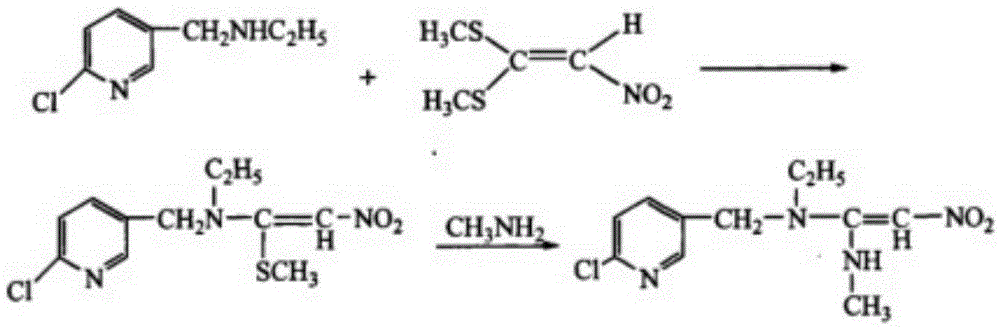
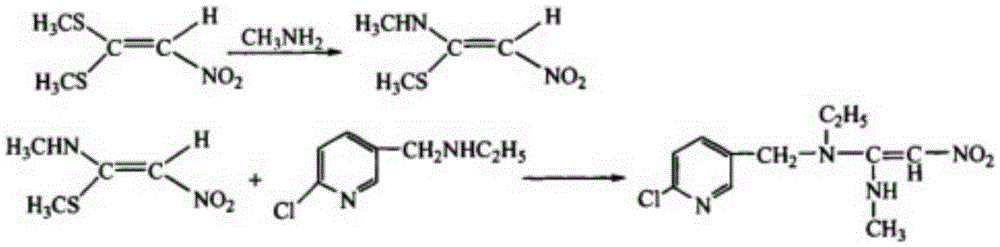
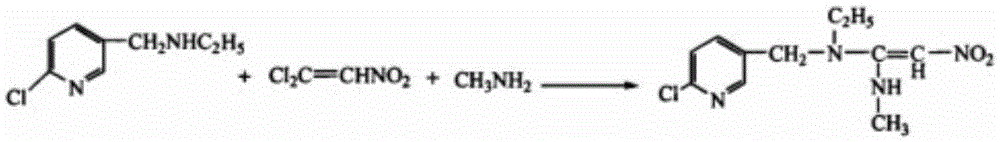Improved preparation method of nitenpyram
A kind of technology of nitenpyram and ethylamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of compound (b) 1,1,1-trichloro-2-nitroethane
[0041] 1. In a 5000L glass-lined reactor, add 2231kg of 36% hydrochloric acid, cool below 10°C, add 978kg of sodium nitrate in batches, stir for 20min, and then add 3kg of tetrabutylammonium bromide;
[0042] 2. Add 970kg of vinylidene chloride dropwise, control the temperature at 10°C, and keep warm for 4 hours after the addition;
[0043] 3. After the reaction is completed, stand still for 30 minutes and separate into layers;
[0044] 4. Add 1000kg of tap water to the organic layer and stir for 30min, then stand still for 30min to separate the layers to obtain 1606.5kg of compound (b) 1,1,1-trichloro-2-nitroethane, GC=90%, molar yield 0.9;
[0045] Preparation of compound (c) 1,1-dichloro-2-nitroethylene
[0046] 1. In a 5000L glass-lined kettle, add 1606.5kg of the obtained compound (b), add 1000kg of tap water, and raise the temperature by 30-40°C;
[0047] 2. Then add 2100kg 8% NaHCO dropwise 3 About...
Embodiment 2
[0068] Preparation of compound (b) 1,1,1-trichloro-2-nitroethane
[0069] 1. In a 5000L glass-lined reactor, add 2231kg of 36% hydrochloric acid, cool below 10°C, add 978kg of sodium nitrate in batches, stir for 20min, and then add 3kg of tetrabutylammonium bromide;
[0070] 2. Add 970kg of vinylidene chloride dropwise, control the temperature at 20°C, and keep warm for 3 hours after the addition;
[0071] 3. After the reaction is completed, stand still for 30 minutes and separate into layers;
[0072] 4. Add 1000kg of tap water to the organic layer and stir for 30min, then stand still for 30min to separate the layers to obtain 1606.5kg of compound (b) 1,1,1-trichloro-2-nitroethane, GC=90%, molar yield 0.9;
[0073] Preparation of compound (c) 1,1-dichloro-2-nitroethylene
[0074] 1. In a 5000L glass-lined kettle, add 1606.5kg of the obtained compound (b), add 1000kg of tap water, and raise the temperature by 30-40°C;
[0075] 2. Then add 2100kg 8% NaHCO dropwise 3 About...
Embodiment 3
[0096] Preparation of compound (b) 1,1,1-trichloro-2-nitroethane
[0097] 1. In a 5000L glass-lined reactor, add 2231kg of 36% hydrochloric acid, cool below 10°C, add 978kg of sodium nitrate in batches, stir for 20min, and then add 3kg of tetrabutylammonium bromide;
[0098] 2. Add 970kg of vinylidene chloride dropwise, control the temperature at 25°C, and keep warm for 3 hours after the addition;
[0099] 3. After the reaction is completed, stand still for 30 minutes and separate into layers;
[0100] 4. Add 1000kg of tap water to the organic layer and stir for 30min, then stand still for 30min to separate the layers to obtain 1606.5kg of compound (b) 1,1,1-trichloro-2-nitroethane, GC=90%, molar yield 0.9;
[0101] Preparation of compound (c) 1,1-dichloro-2-nitroethylene
[0102] 1. In a 5000L glass-lined kettle, add 1606.5kg of the obtained compound (b), add 1000kg of tap water, and raise the temperature by 30-40°C;
[0103] 2. Then add 2100kg 8% NaHCO dropwise 3 About...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com