Method for compounding 1-(2-Chloroethoxy)propane
A technology of propoxy ethyl chloride and n-propyl ether, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high price, low product yield, cumbersome process, etc., and achieve production cost Low production rate, high product yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
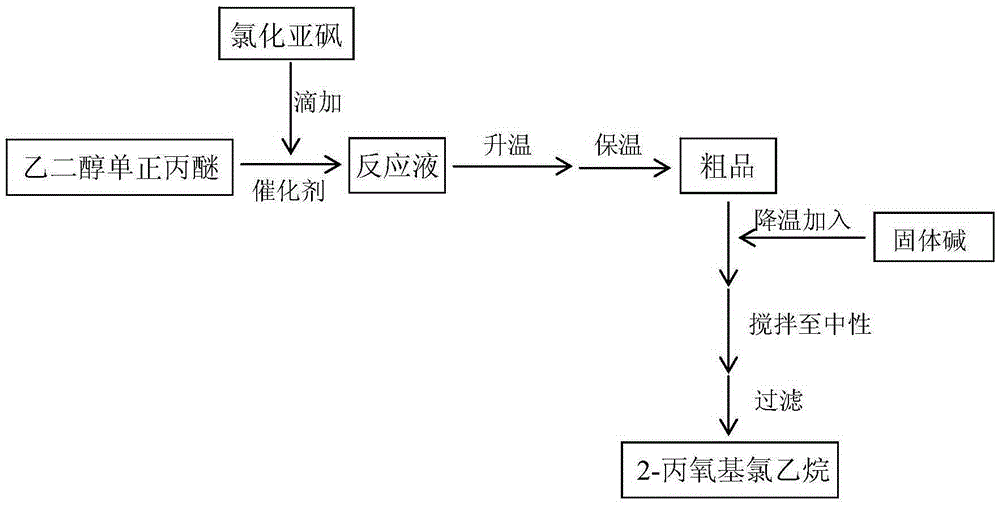
[0029] Take 100g of ethylene glycol mono-n-propyl ether and 0.1g of dodecyltrimethylammonium chloride respectively and add them to the reaction vessel with a reflux condenser, add 125.66g of thionyl chloride to the constant pressure dropping funnel, and heat up to 35 ℃, start stirring, slowly add thionyl chloride dropwise to the reaction vessel, the reaction process releases sulfur dioxide and hydrogen chloride gas, control the rate of thionyl chloride addition to keep the temperature of the reaction solution at 55±5°C.
[0030] After the dropwise addition of thionyl chloride, gradually raise the temperature to 90°C according to the reaction gas release rate until no gas is released, and continue to keep warm for 2h to obtain the crude product; cool the crude product to 50°C, directly add solid sodium hydroxide and stir, and thoroughly dissolve the thionyl chloride Decompose to obtain 116.19 g of 2-propoxyl chloroethane. After testing, the chromatographic content of the product...
Embodiment 2
[0032] Take 100g of ethylene glycol mono-n-propyl ether and 0.3g of benzyltriethylammonium chloride into the reaction vessel with a reflux condenser, add 131.4g of thionyl chloride into the constant pressure dropping funnel, and heat up to 40°C. Start stirring, and slowly add thionyl chloride dropwise to the reaction vessel. During the reaction, sulfur dioxide and hydrogen chloride gas are released, and the rate of addition of thionyl chloride is controlled to keep the temperature of the reaction solution at 60±5°C.
[0033] After the dropwise addition of thionyl chloride is completed, gradually raise the temperature to 95°C according to the reaction gas release rate until no gas is released, and continue to keep warm for 3h to obtain the crude product; cool the crude product to 40°C, directly add solid sodium hydroxide and stir, and thoroughly dissolve the thionyl chloride Decompose to obtain 116.2 g of 2-propoxyl ethyl chloride. After testing, the chromatographic content of t...
Embodiment 3
[0035] Get 100g ethylene glycol mono-n-propyl ether, 0.5g methoxymethyltriphenylphosphine chloride respectively and add in the reaction vessel with reflux condenser, add 137.1g thionyl chloride into the constant pressure dropping funnel, heat up to At 45°C, start stirring, and slowly add thionyl chloride dropwise to the reaction vessel. During the reaction, sulfur dioxide and hydrogen chloride gas are released, and the rate of addition of thionyl chloride is controlled to keep the temperature of the reaction solution at 60±5°C.
[0036] After the dropwise addition of thionyl chloride, gradually raise the temperature to 100°C according to the reaction gas release rate until no gas is released, and continue to keep warm for 4h to obtain the crude product; cool the crude product to 30°C, directly add solid sodium hydroxide and stir, and thoroughly dissolve the thionyl chloride Decompose to obtain 116.08g of 2-propoxyl chloroethane. After testing, the chromatographic content of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com