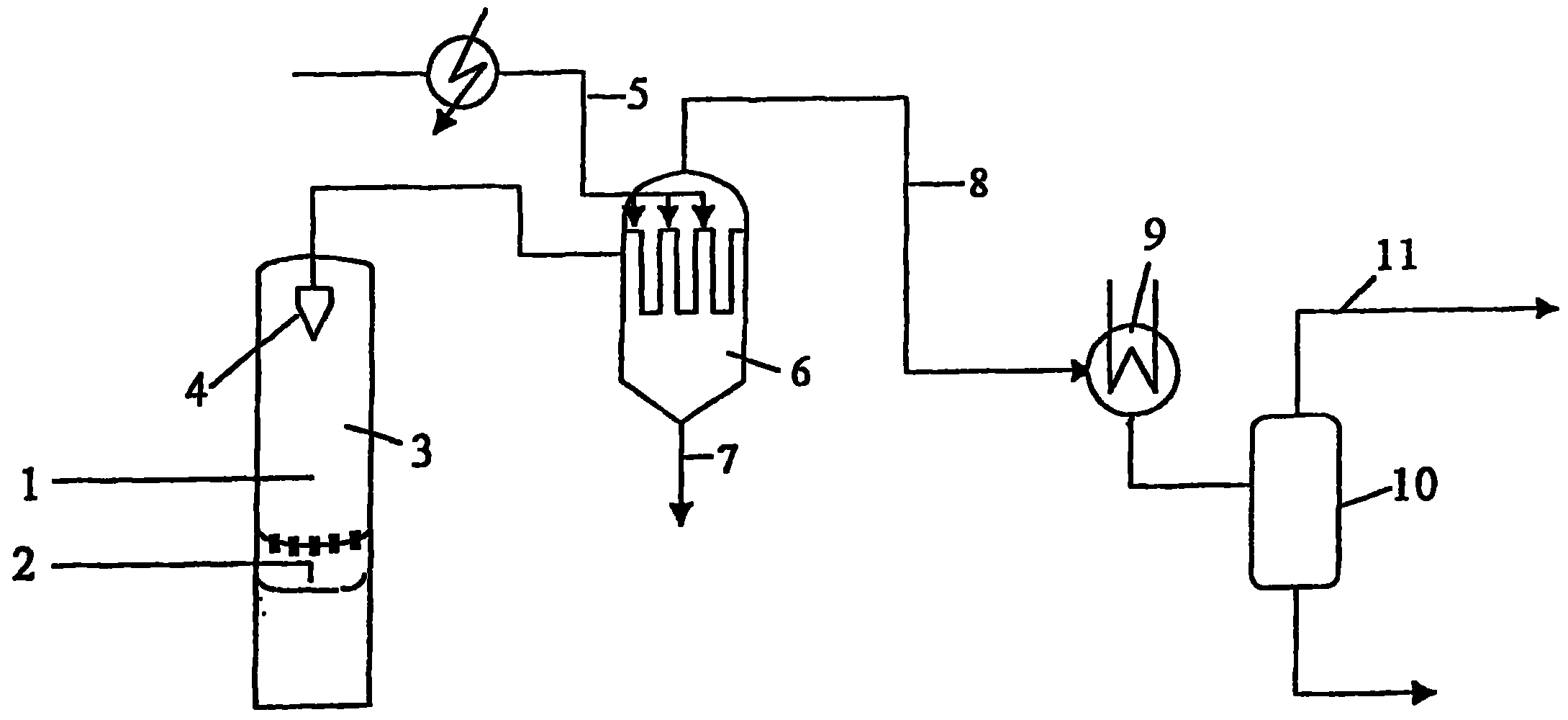
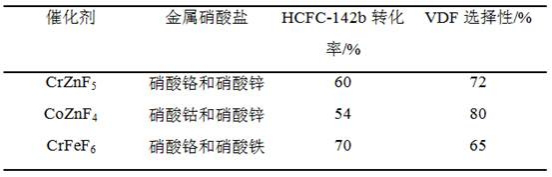
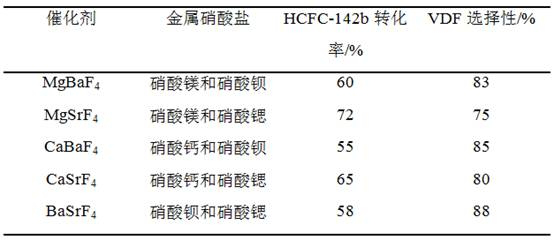
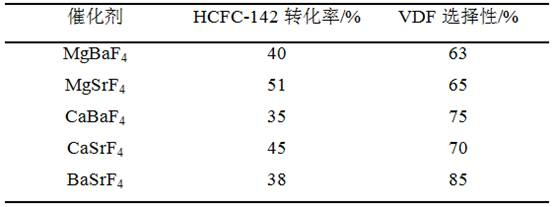
Patents
Literature
46 results about "Ethylene Chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethylene chloride (C2H4Cl2), also called ethylene dichloride or 1,2-dichloroethane, a colourless, toxic, volatile liquid having an odour resembling that of chloroform. It is denser than water, and it is practically insoluble in water. Ethylene chloride is produced by the reaction of ethylene and chlorine.
Waterproof and anticorrosive paint special for marine ships
InactiveCN106318105AAccelerated corrosionImprove water resistanceRubber derivative coatingsAnti-corrosive paintsDimethylaniline N-oxideDefoamer
The invention discloses a waterproof and anticorrosive paint special for marine ships. The paint comprises 60-85wt% of a component A and 15-40wt% of a component B. The component A comprises the following raw materials: epoxy resin, chlorinated rubber, an ethylene chloride-vinyl acetate copolymer, organic fluorine modified acrylic resin, a silane coupling agent, zinc powder, mica powder, iron oxide red, ceramic micro-powder, molybdenum disulfide, propylene glycol butyl ether, propylene glycol methyl ether acetate, a dispersing agent, an organic silicon defoamer, a leveling agent, methyl isobutyl ketone and n-butanol; and the component B includes the following raw materials: poly 2, 3-dimethylaniline modified montmorillonite, a curing agent, ethyl acetate, and butyl acetate. The waterproof and anticorrosive paint special for marine ships provided by the invention has excellent waterproof performance and corrosion resistance, also has excellent impact resistance, abrasion resistance and weather resistance, has good comprehensive performance when used in ship engineering, and has long service life.
Owner:ANHUI KAILIN ADVANCED MATERIAL CO LTD
Wear-resistant oil-resistant flame-retardant polyvinyl chloride wire and cable material and preparation method thereof
The invention relates to the field of a polyvinyl chloride material used for making wire and cable and discloses a wear-resistant oil-resistant flame-retardant polyvinyl chloride wire and cable material. The formula of the material provided by the invention contains the following ingredients, by weight, 100 parts of polyvinyl chloride (PVC), 55-75 parts of one or more substances selected from trimellitate, polyester and an epoxy plasticizer, 5-10 parts of one or more substances selected from a compound stabilizer and an epoxy compound, 30-50 parts of one or more substances selected from antimonous oxide, magnesium hydroxide, a composite flame retardant and calcium carbonate, 10-35 parts of one or more substances selected from ethylene chloride and nitrile rubber, 0.5-0.8 part of one or more substances selected from stearic acid, polyethylene wax and a composite lubricant and 1-2 parts of color masterbatch. The wire and cable prepared by the technical scheme can have effects of wear resistance, oil resistance and flame retardation.
Owner:HUIZHOU LTK ELECTRONICS CABLE +3
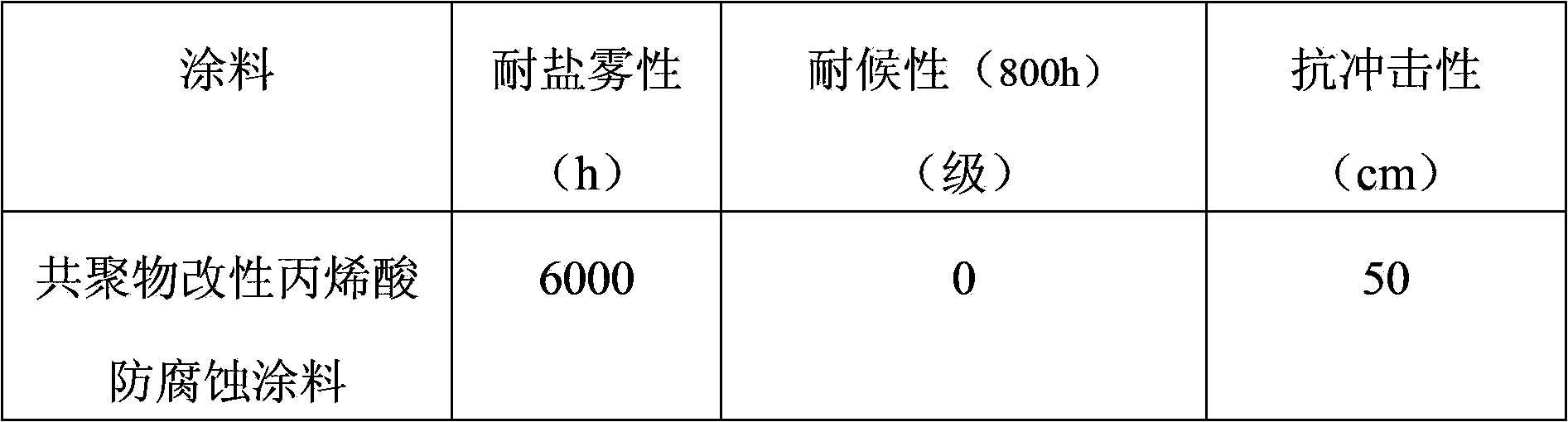
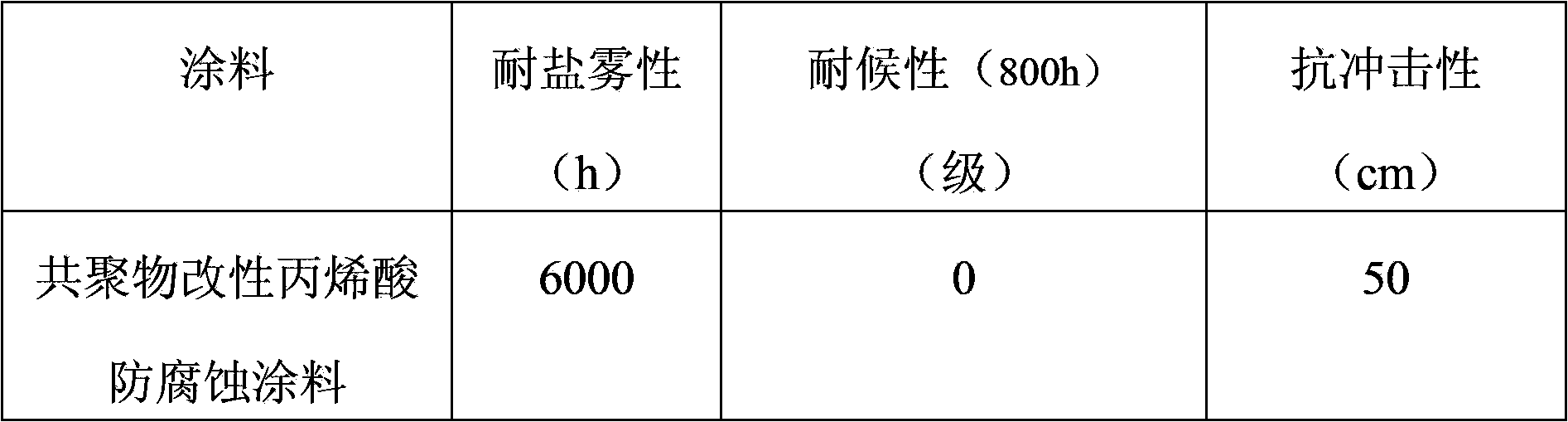
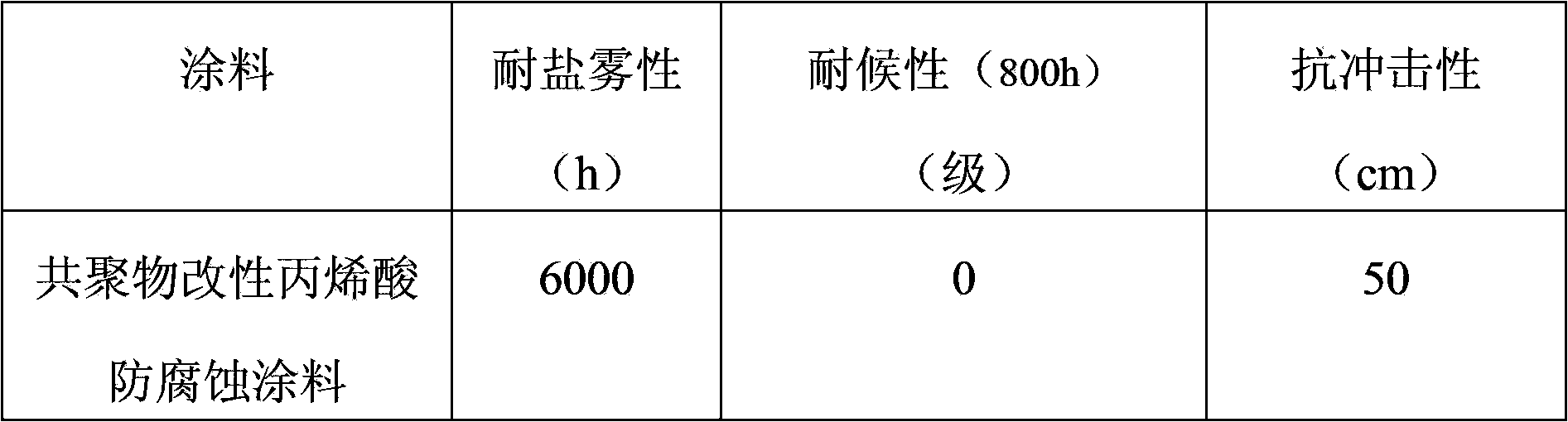
Copolymer modified acrylic acid anticorrosive coating and manufacture method thereof
InactiveCN103450756AImprove adhesionImprove impact resistanceAnti-corrosive paintsCellulose acetate-butyratePolymer chemistry
The invention relates to a copolymer modified acrylic acid anticorrosive coating and a manufacture method thereof. The copolymer modified acrylic acid anticorrosive coating comprises thermoplastic acrylic resin, an ethylene chloride-vinyl acetate copolymer, xylene, n-butanol, n-butyl acetate, cellulose acetate butyrate, petroleum resin, rutile-type titanium dioxide, powdered steatile, mica powder, a dispersant, an adhesion improver, a scratch-resistant agent, an antifoaming agent without organosilicone, a flatting agent, an anti-settling agent and an ultraviolet light absorbent. The manufacture method comprises steps of mixing, high-speed dispersion and grinding; and during usage, a film is formed along with volatilization of the solvents. The coating has the advantages of good weatherability, long salt spray resistance, resistance to erosion of a plurality of chemical media, can be prepared into a plurality of colors and is convenient for construction; and the copolymer modified acrylic acid anticorrosive coating can be applied to anticorrosion of steel objects including ocean platform, ship deck, bridge, building, pipeline and storage tank.
Owner:TIANJIN BO XING ENG SCI & TECH LIMITED COMPANY OF CNPC +1
Antimony-free flame-retardant polyvinyl chloride wire and cable material and preparation method thereof
InactiveCN104403224AMeet the requirements of combustion classMeet the requirements of the combustion levelPlastic/resin/waxes insulatorsPolymer scienceAluminium hydroxide
The invention relates to the field of a polyvinyl chloride wire and cable material, especially to an antimony-free flame-retardant polyvinyl chloride wire and cable material. The formula of the wire and cable material contains the following ingredients, by weight, a. 100 parts of polyvinyl chloride (PVC); b. 45-55 parts of one or more substances selected from aliphatic dibasic acid ester, trimellitate, polyester and an epoxy plasticizer; c. 5-11 parts of one or more substances selected from a compound stabilizer and an epoxy compound; d. 10-50 parts of one or more substances selected from hydrous zinc borate, magnesium hydroxide, aluminium hydroxide, huntite and a compound flame retardant; e. 4-25 parts of one or more substances selected from kaolin, ceramic microbead and silica-alumina mineral powder; f. 0-6 parts of zero or more substances selected form ethylene chloride and acrylate; g. 0.5-0.8 part of one or more substances selected from stearic acid, polyethylene wax and a compound lubricant; and h. 1-2 parts of color masterbatch. By the technical scheme, latent personal safety threat existing due to antimony can be minimized, pollution is reduced, and the wire and cable material is more environmentally-friendly and has higher safety.
Owner:HUIZHOU LTK ELECTRONICS CABLE +3
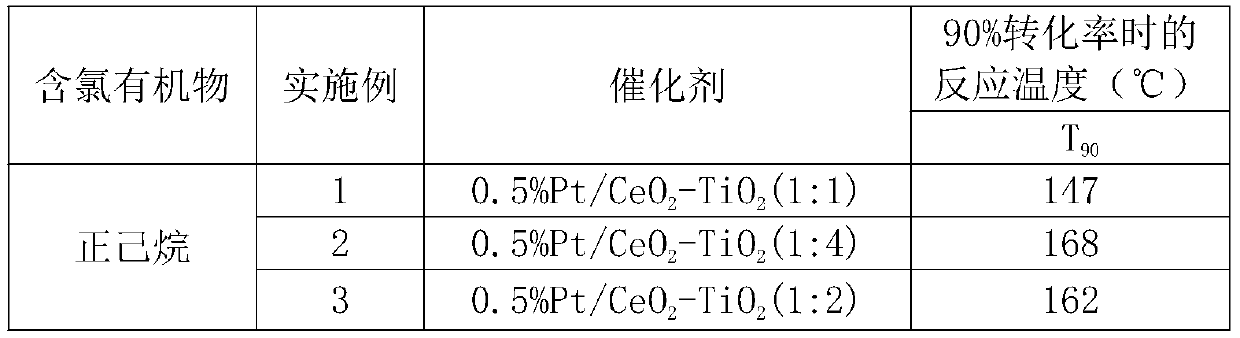
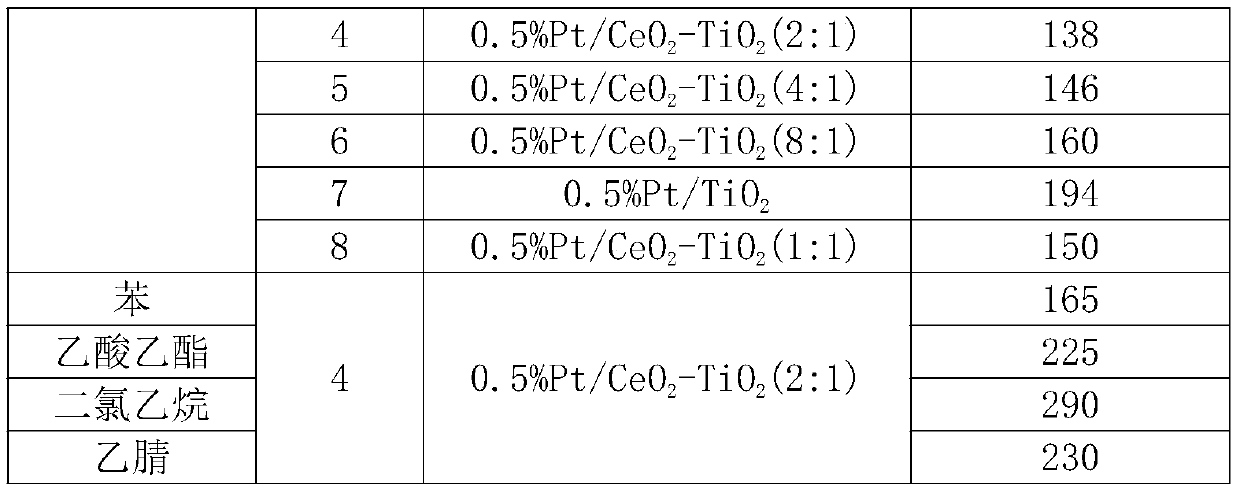
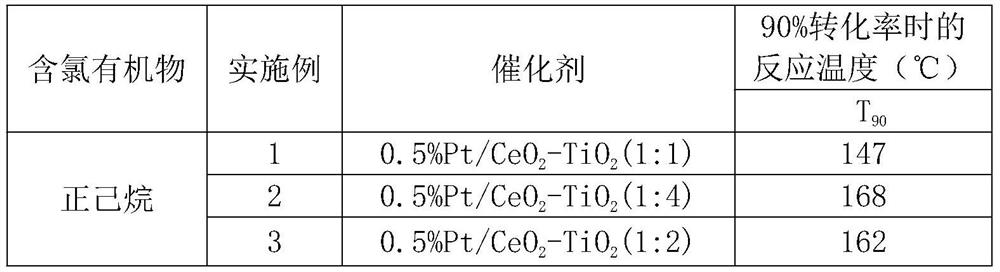
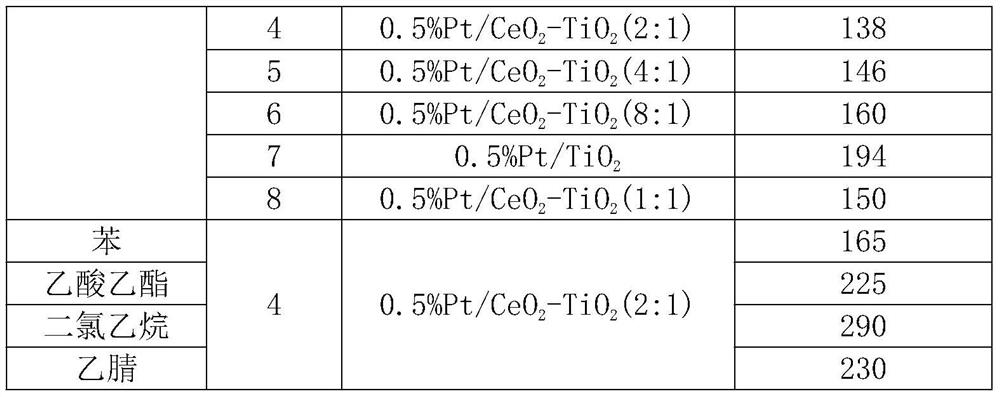
Preparation method of efficient and stable Pt/CeO2-TiO2 catalyst for oxidative degradation of VOCs
ActiveCN111408371ALow costSimple preparation processCatalyst carriersCatalyst activation/preparationTitanium chloridePtru catalyst
The invention provides a preparation method of an efficient and stable Pt / CeO2-TiO2 catalyst for oxidative degradation of VOCs (Volatile Organic Chemicals). Cheap cerous chloride or cerous nitrate, titanium tetrachloride and a small amount of chloroplatinic acid are used as raw materials; a CeO2-TiO2 composite oxide carrier is prepared by adopting a precipitation method, Pt is loaded by adopting an electron-free deposition method to prepare the Pt / CeO2-TiO2 catalyst, and the optimal molar ratio of CeO2 to TiO2 in the catalyst is 4: 1-1: 1. The catalyst is simple in preparation process and lowin cost. The catalyst prepared by the invention is particularly suitable for catalytic combustion of organic waste gas containing chlorine VOCs or non-chlorine VOCs containing benzene, normal hexane and the like; the complete oxidation temperature of the catalyst to dichloroethane and n-hexane is only about 290 DEG C and 140 DEG C respectively, the catalytic activity is not reduced when dichloroethane and n-hexane coexist, and the catalyst has excellent low-temperature catalytic oxidation degradation activity and stability and good application prospect.
Owner:ZHEJIANG UNIV
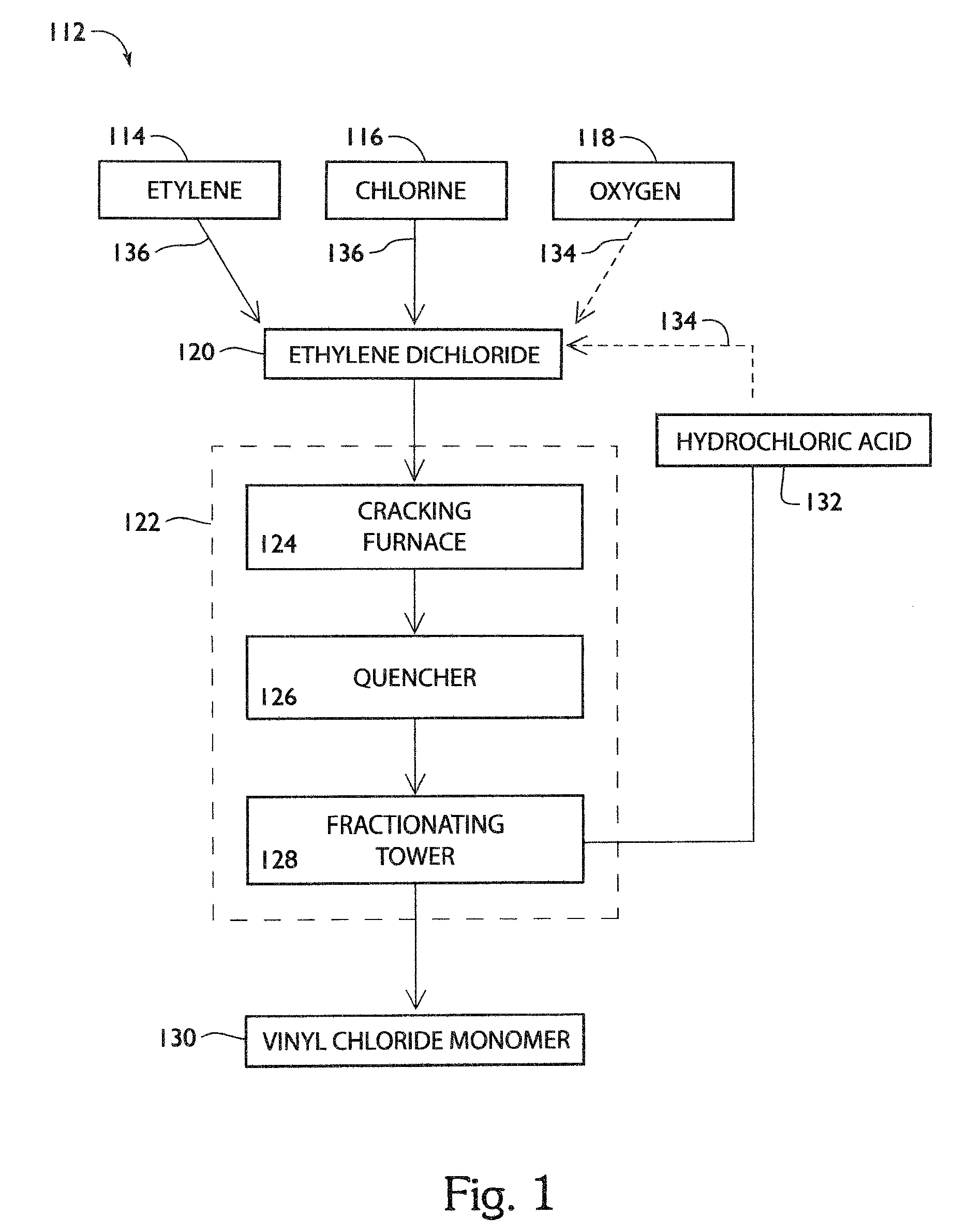
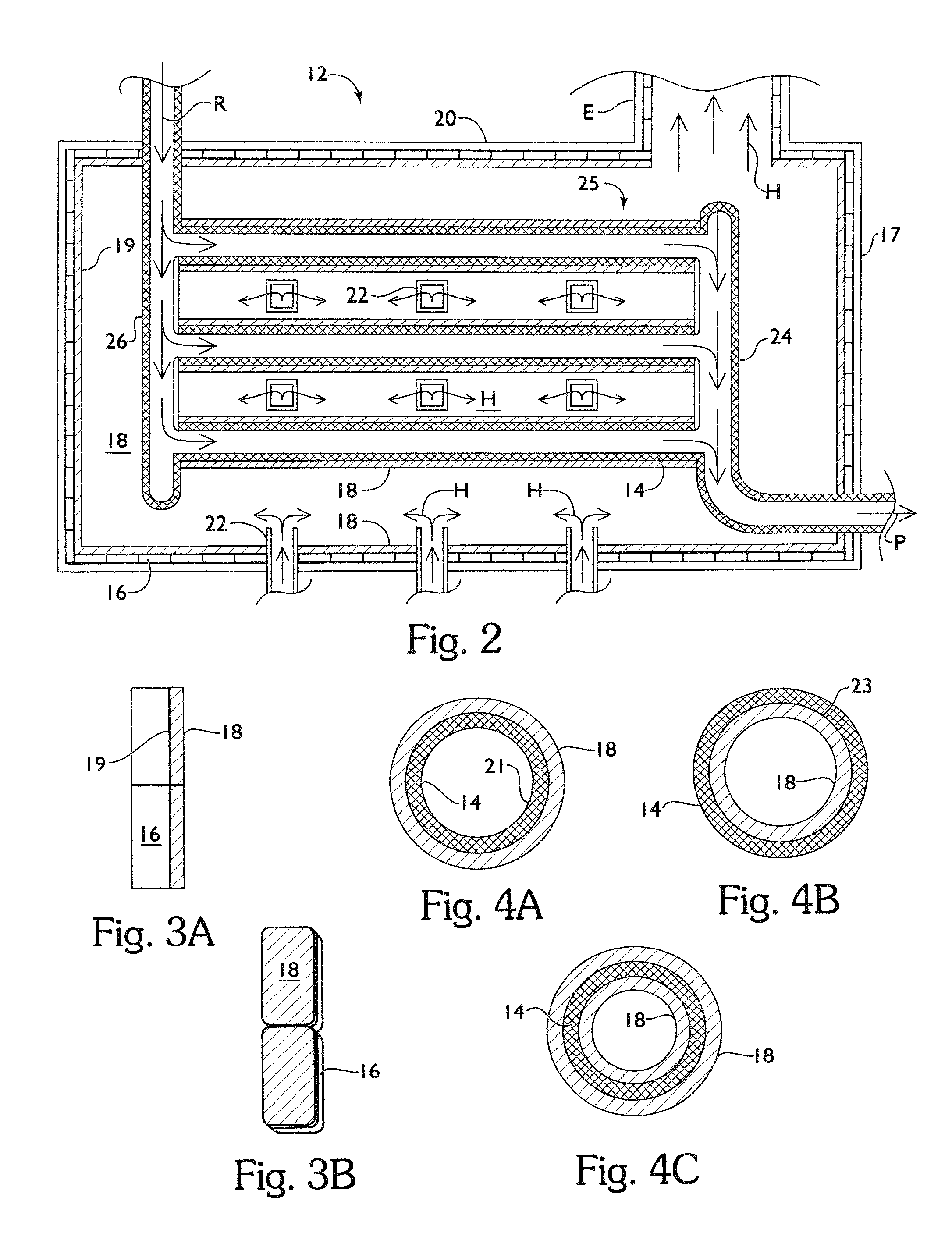
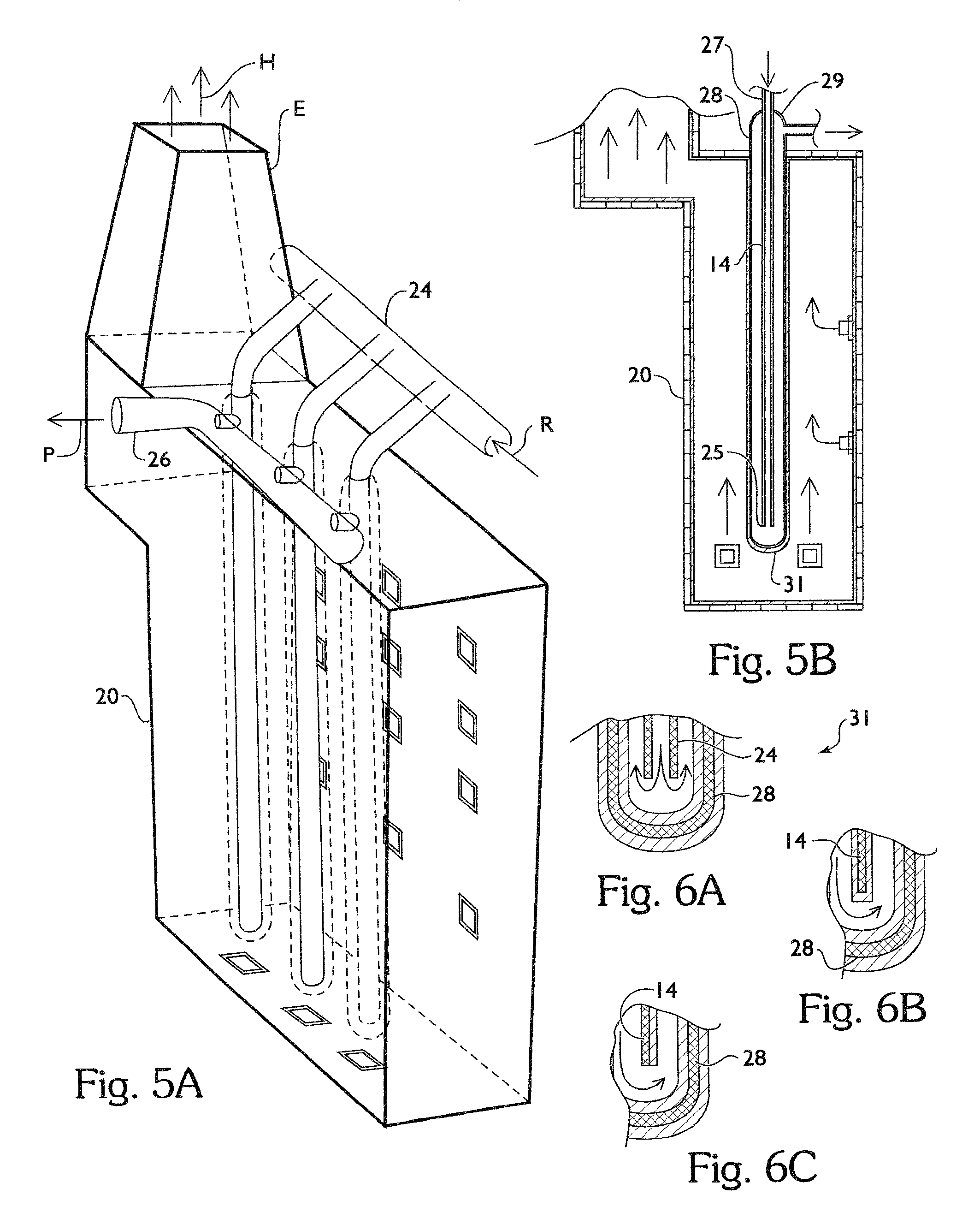
Process and apparatus for production of vinyl chloride monomer
ActiveUS7968756B2Increase volumeReduce maintenancePreparation by hydrogen halide split-offChemical/physical/physico-chemical processesColloidal silicaAdhesive
Process and apparatus to form vinyl chloride monomer from ethylene dichloride in a cracking furnace, including a firebox chamber having a thermal protective layer disposed on refractory walls and / or process tubes disposed within the chamber, a quencher to form vinyl chloride monomer, and fractionator separate products. The thermal protective layer which contains an inorganic adhesive for metal / alloy tubes or colloidal silica and / or colloidal alumina for refractory walls or ceramic tubes, a filler, and one or more emissivity agents.
Owner:WESSEX
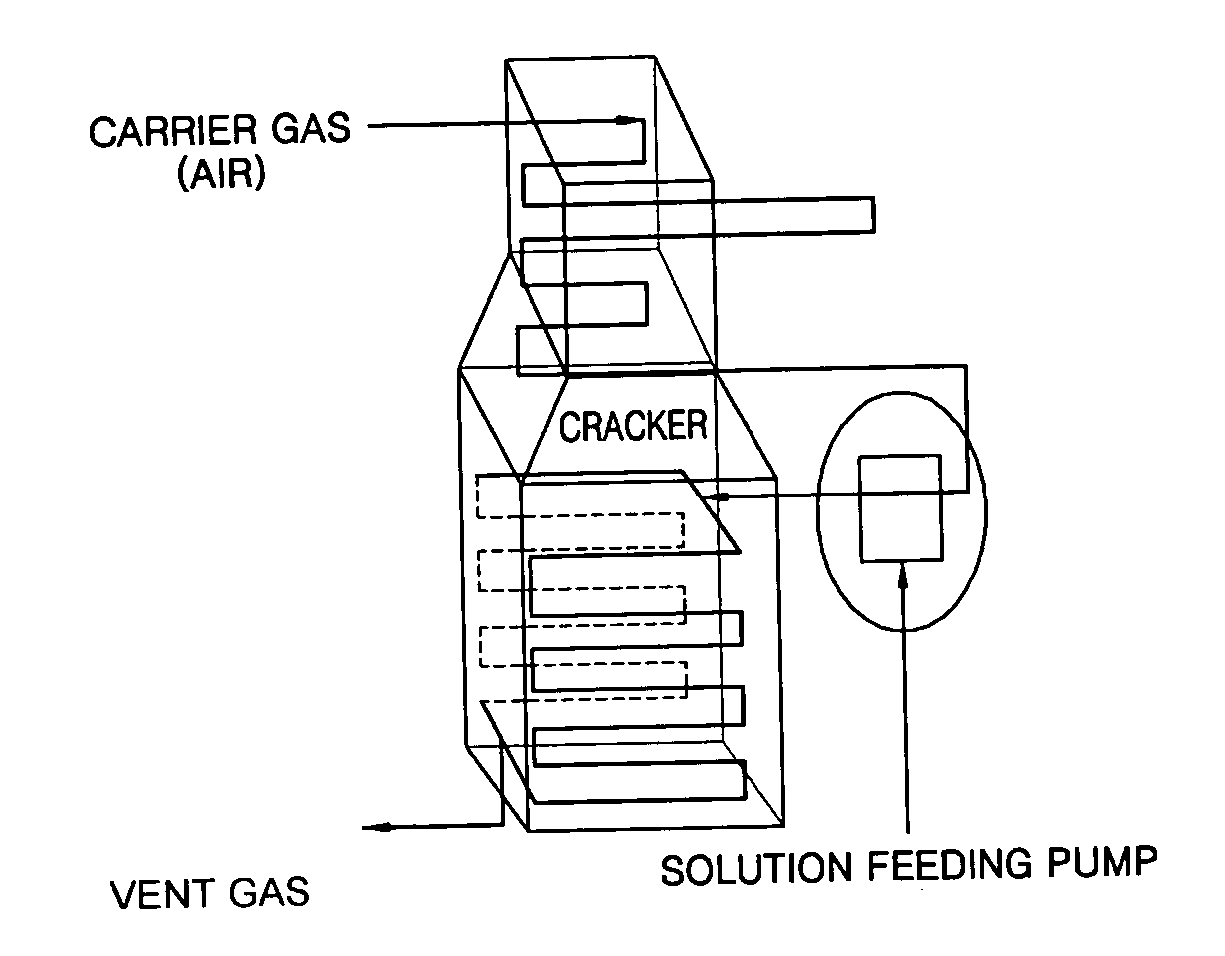
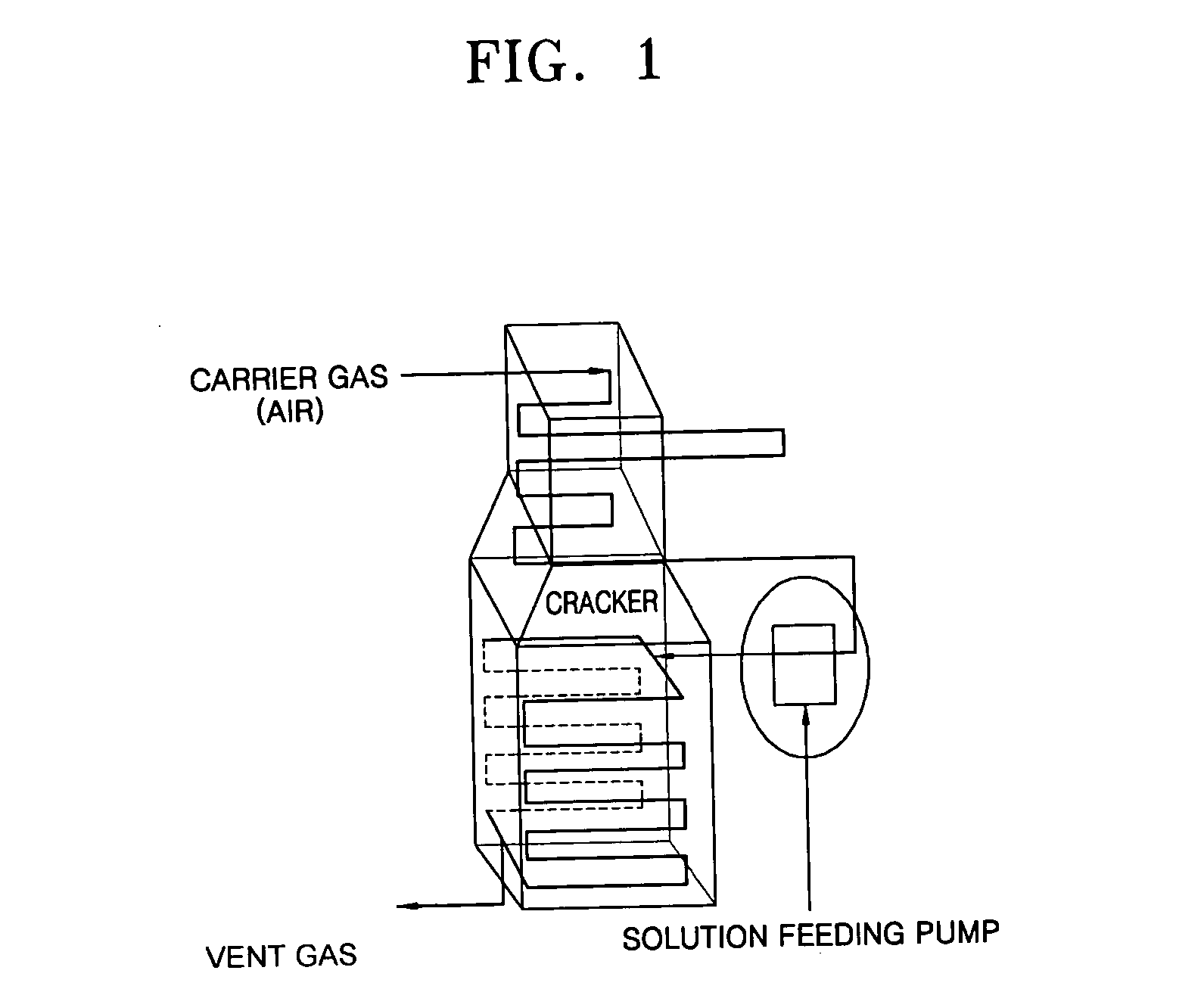
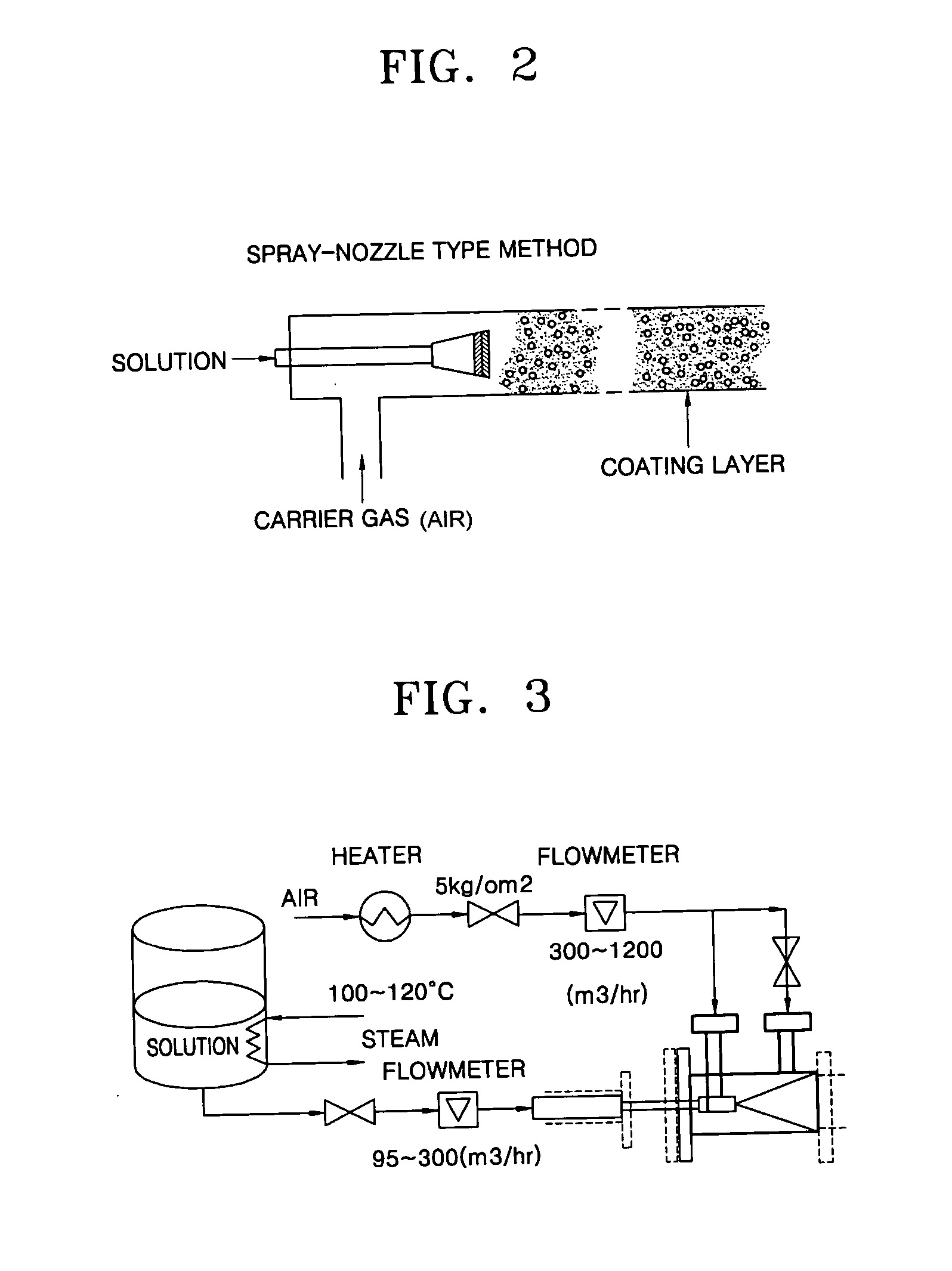
Coating film for inhibiting coke formation in ethylene dichloride pyrolysis cracker and method of producing the same
InactiveUS20060127700A1Inhibition is effectiveSpeed up the conversion processThermal non-catalytic crackingOven incrustations prevention/removalEthylene DichlorideBoron
Provided is a coating film that inhibits the formation of coke in an ethylene dichloride to a vinyl chloride monomer pyrolysis cracker and a method of producing the coating film. The coke formation, which occurs during a pyrolysis reaction, is inhibited by coating a boron compound on a heat-transfer surface of the cracker. As a result, the amount of coke generated when a coke formation-inhibiting material is coated is decreased by 50% or greater than that when the coke formation-inhibiting material is not coated. In this case, however, the ethylene chloride conversion and the selectivity to a vinyl chloride monomer during the pyrolysis reaction are not affected. Accordingly, the efficiency of the pyrolysis cracker can be maximized.
Owner:LG CHEM LTD
Method of inhibiting coke formation in ethylene dichloride pyrolysis cracker
InactiveUS20060129007A1Efficiently inhibiting formation of cokeEfficiently inhibits coke formationThermal non-catalytic crackingPreparation by hydrogen halide split-offEthylene DichlorideBoron
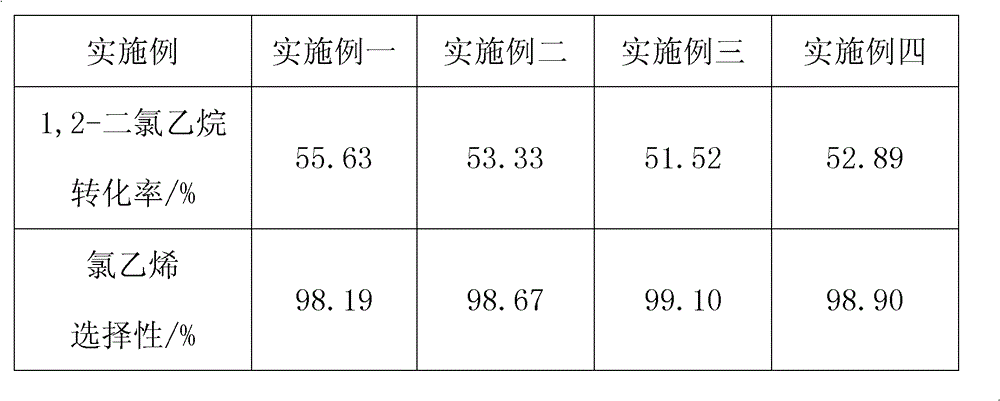
Provided is a method of inhibiting the formation of coke in an ethylene dichloride to vinyl chloride monomer pyrolysis cracker. The coke formation, which occurs during an ethylene dichloride pyrolysis reaction, is inhibited by contacting a heat-transfer surface of the cracker and a boron compound. The amount of coke generated when a coke formation-inhibiting material is used is 50% or less of that when a coke formation-inhibiting material is not used. In this case, however, the ethylene chloride conversion and the selectivity to a vinyl chloride monomer during the pyrolysis reaction are not affected. Accordingly, the efficiency of the pyrolysis cracker can be maximized.
Owner:LG CHEM LTD
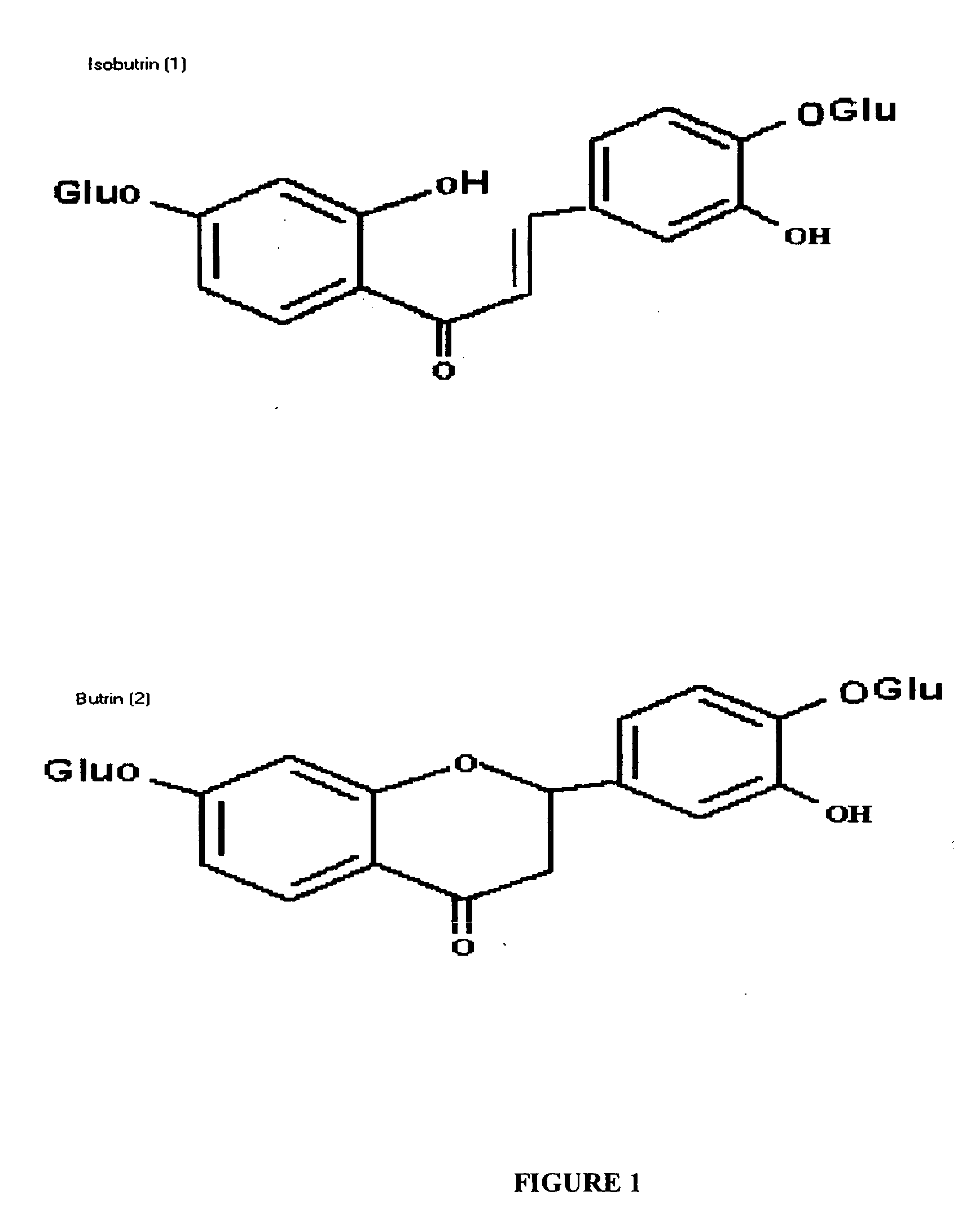
Pharmaceutical composition useful for the treatment of hepatocellular carcinoma
The present invention relates to anticancer activity against hepatocellular carcinoma of an extract and fraction isolated from flowers of Butea monosperma. Particularly, this invention relates to anticancer activity against hepatocellular carcinoma of a composition containing markered flavonoid glycosides such as butrin and isobutrin in the range of 2 to 9% by weight, isolated from the flowers of Butea monosperma by extracting the flowers with polar solvent like ethanol, methanol, aqueous ethanol or water, removing fatty non-polar constituents by triturating the extract with solvents such as ethylene chloride, methylene chloride, chloroform or ethyl acetate, suspending the residue in water, extracting with n-butanol and freeze drying the aqueous part.
Owner:COUNCIL OF SCI & IND RES +1
Flame retardant polyurethane spray coating foamed plastic
ActiveCN102558484BReduce intensityImprove heat resistancePolyurea/polyurethane coatingsPhosphoric Acid EstersPolymer science
The invention discloses a flame retardant polyurethane spray coating foamed plastic, which is composed of a combination white material and isocyanate with mass ratio as 1: 1-1.2. The combination white material includes the following components by mass: 65 to 75 parts of trihydroxyl-amino resin, 5 to 15 parts of trihydroxyl phosphate, 8 to 20 parts of poly-phosphate amine, 10 to 15 parts of glycerin, 2.5 to 3 parts of silicon-carbon bond non-hydrolysis polysiloxane- polyether copolymer, 0.6 to 0.8 part of water, 0.25 to 0.3 part of N,N,N,N,N-pentamethyl-diethylenetriamine, 0.8 to 1 part of dimethylcyclohexylamine, 1.8 to 2 parts of dipropylene glycol solution of triethylene diamine of 33+ / -1Wt.%, 0.15 to 0.2 part of dibutyltin dilaurate catalyst, 3 to 3.5 parts of hexahydro-triazine, 11 to 15 parts of fatty alcohol-polyoxyethylene ether, 25 to 35 parts of 4(2-chloride ethyl) diethylene-ether-diphosphate and 30 to 38 parts of fluorine ethylene dichloride. The flame retardant polyurethane spray coating foamed plastic is good in heat resistance, fire penetration resistance and flexibility and high in strength. According to the detection, the inflaming retarding grade of the product can reach the B grade according to GB / T8624-2006 standard, so that the flame retardant polyurethane spray coating foamed plastic can be used in various fireproof building fields.
Owner:JIANGSU CHANGNENG ENERGY SAVING NEW MATERIALS SCI & TECH
Method of inhibiting coke formation in ethylene dichloride pyrolysis cracker
InactiveUS7132577B2Efficiently inhibiting formation of cokeEfficiently inhibits coke formationThermal non-catalytic crackingPreparation by hydrogen halide split-offEthylene DichlorideBoron
Provided is a method of inhibiting the formation of coke in an ethylene dichloride to vinyl chloride monomer pyrolysis cracker. The coke formation, which occurs during an ethylene dichloride pyrolysis reaction, is inhibited by contacting a heat-transfer surface of the cracker and a boron compound. The amount of coke generated when a coke formation-inhibiting material is used is 50% or less of that when a coke formation-inhibiting material is not used. In this case, however, the ethylene chloride conversion and the selectivity to a vinyl chloride monomer during the pyrolysis reaction are not affected. Accordingly, the efficiency of the pyrolysis cracker can be maximized.
Owner:LG CHEM LTD
Outdoor compound enhanced ethylene chloride HFCM pipe and preparation method thereof
InactiveCN103937135ADoes not show brittlenessExcellent low temperature toughnessAging resistanceToughness
The invention discloses an outdoor compound enhanced ethylene chloride HFCM pipe and a preparation method thereof. The pipe comprises the following components in parts by weight: 100 parts of resin, 3-4 parts of ACR (Acrylics) processing assistant acrylate, 8-10 parts of MBS (Methyl methacrylate-Butadiene-Styrene) impact modifier, 2-3 parts of Q-108 flexibilizer, 5-10 parts of filler nano calcium carbonate, 4-5 parts of stabilizer, 0.5-1 part of lubricant, and 2 parts of pigments and antioxidant. The preparation method comprises the following steps: dosing; kneading at high speed; cooling and stirring; and plastifying and extruding. The pipe disclosed by the invention is low in preparation cost and free from brittleness. The pipe is good in low-temperature toughness resistance, aging resistance, flame retardance and insulation and compressive property. The tubular product is convenient to connect and long in service life.
Owner:FUYANG STEEL IND OF KEWEI CO LTD
Alkyd rust-proof coating for metal
InactiveCN106065269ASmooth appearanceEasy to prepareAnti-corrosive paintsPolyester coatingsSlurryKetone
The invention discloses an alkyd rust-proof coating for metal, which includes the raw materials of: alkyd resin, aluminum powder slurry, rosin-modified phenolic resin, hydroquinone, methyl-ethyl ketone, tung oil, vinyl acetate, ethylene chloride, linseed oil and azodiisobutyronitrile. The coating can form a flat and smooth paint film. The coating is 100% in adhesion force, is 10-14 h in surface drying time and 16-20 h in hard drying time, is 50-90 N / mm in impact strength, is 220-260 g / m<2> in coverage force, is 0.3-0.7 in hardness and is 20-40 s in surface viscosity. The coating is simple is preparation method, is prepared from easy-to-obtained raw materials, can be widely produced and can continuously replace materials in the prior art.
Owner:朱小龙
The preparation method of vinyl sulfate
The invention discloses a preparation method of vinyl sulfate, and belongs to the technical field of electrolyte additives for lithium ion batteries. The method comprises: 1) adding ethylene glycol dropwise into thionyl chloride, raising the temperature to 50-80° C. for 2-4 hours, and performing vacuum treatment to obtain a reaction solution containing vinyl sulfite; 2) adding The reaction solution of vinyl sulfite is rectified and purified under vacuum conditions; 3) Mix vinyl sulfite with a purity greater than 99.2% and dichloroethane, add sodium hypochlorite solution dropwise to the mixture, and stir after the addition is completed, Stand still to obtain the organic phase; 4) Adjust the pH value of the organic phase to 7~8, then add the catalyst, dropwise add sodium hypochlorite solution for oxidation reaction, keep warm for 1~1.5h, stand still to separate the liquid, and obtain the crude product of vinyl sulfate; 5) The crude vinyl sulfate is dehydrated, recrystallized and dried to obtain vinyl sulfate. The method provided by the invention has high yield and high purity of vinyl sulfate.
Owner:DONGYING HI TECH SPRING CHEM IND
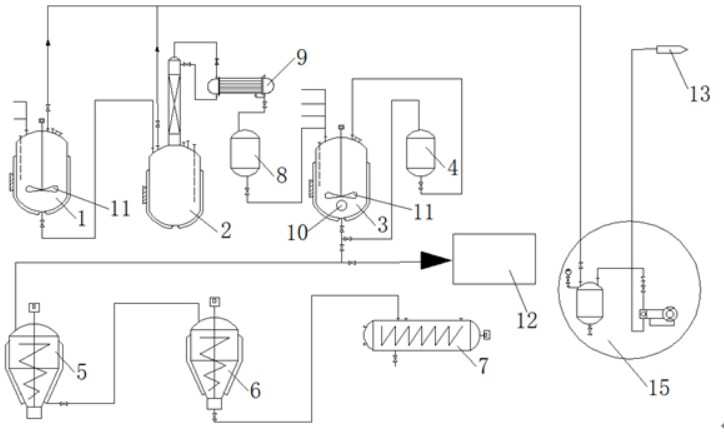
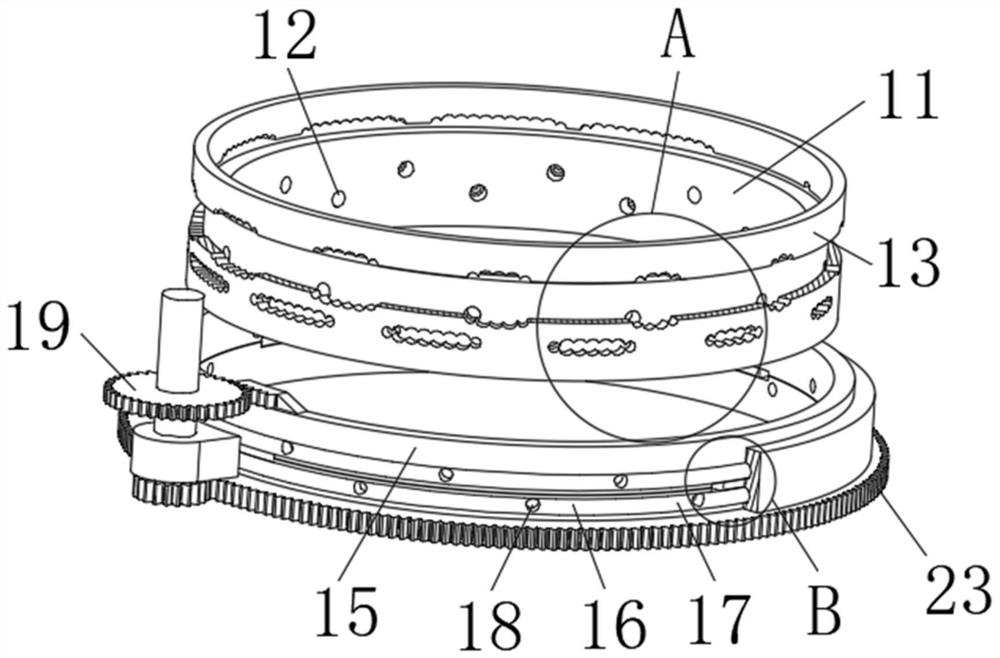
Tower-type device for decomposing ethylene chloride and manufacturing method of key components thereof
ActiveCN105819609AEasy to operateHigh degree of automationWater contaminantsWater aerationTreatment effectExhaust pipe
The invention discloses a tower-type device for decomposing ethylene chloride and a manufacturing method of key components thereof .The tower-type device is composed of a thermometer, a heating device, a central control system, a top end gas exhaust pipe, a sewage return pipe, a water storage pond, a primary aeration device, a secondary aeration device, a middle gas exhaust pipe, a padding device, an ethylene chloride concentration detector, a water collection pond, a water draining pipe and a purified water conveying pipe .The water storage pond is of a cylindrical structural body .The side wall of the water storage pond is made of stainless steel materials .The heating device is connected with the central control system through a wire .The water storage pond is connected to the upper end of the sewage return pipe .The lower end of the sewage return pipe is connected with the water collection pond .The primary aeration device is located below the water storage pond .The upper end of the water storage pond and the lower end of the primary aeration device are welded in a seamless mode .The tower-type device has the advantages that operation is easy, the automatic degree is high, and the labor intensity is low; the device utilizes the aeration devices and the padding device for treating sewage, and the treatment effect is good; the good environmental and economic benefits are achieved .
Owner:JIANGSU VOCATIONAL INST OF ARCHITECTURAL TECH
Electromagnetic shielding electro-conductive paint and preparation method thereof
InactiveCN104974609AImprove conductivityHigh transparencyElectrically-conductive paintsElectromagnetic shieldingKetone
The invention discloses an electromagnetic shielding electro-conductive paint and a preparation method thereof. The paint comprises the following raw materials: nickel powder, decanol, dibutyl tin laurate, hydroquinone, ethyl methyl ketone, phenothiazine, vinyl acetate, ethylene chloride, diisobutyl ketone, and azodiisobutyronitrile. The adhesive force is 100%, the surface drying time is 1 to 5 minutes, the hard drying time is 10 to 30 minutes, the pencil hardness is 4 to 8H, the impact strength is 60 to 100 MPa, the wear resistance weight loss is 0.001 to 0.01 g / cm2, the surface resistivity is 0.1 to 0.5 [omega] / cm2, and the surface viscosity is 20 to 40 s.
Owner:苏州洋杰电子有限公司
Concrete adhesive
The invention discloses a concrete adhesive, which is prepared from the following raw materials in parts by weight: 3-8 parts of lithium silicate, 6-8 parts of an enhancer, 7-11 parts of vinylidene chloride, 6-10 parts of dichloroethane, 5-10 parts of acetone, 5-11 parts of imide, 3-10 parts of dibutyl phthalate, 4-9 parts of emulsified asphalt, 6-8 parts of an ethylene-acrylic acid copolymer, 3-6 parts of ester ring aldehyde, 5-10 parts of dimeric epoxy pentadiene, 4-8 parts of aluminum oxide, 9-15 parts of acrylic resin and 6-8 parts of epoxy resin. The concrete adhesive disclosed by the invention has the beneficial effects of high bonding strength, and no loosening or crack, and is capable of effectively restoring concrete.
Owner:张桂华
Environmentally friendly sizing agent for pure cotton yarn
The invention discloses an environmentally friendly slurry for pure cotton yarn, which is made of the following mass ratio raw materials: 50-75% of dried distiller's grain powder, 5-25% of sodium hydroxide solution, 2-10% of triethanolamine, epoxy chlorine Propane 3-8%, butyl acrylate 1-10%. The invention adopts powdered beer dried distiller's grains powder as a raw material for preparing composite slurry, not only effectively utilizes waste protein resources, but also shortens extraction time, improves yarn strength and reduces yarn hairiness.
Owner:SUQIAN YUANYANG BIOTECH
New preparation method of lanoconazole
Methanol and concentrated sulphuric acid are taken as solvent and 2-chloric mandelic acid is taken as raw material for esterification reaction, sodium hydroxide is added to be neutral after full reaction, reducing reaction is carried out with sodium borohydride, diluted hydrochloric acid is used for neutralization and water is added for dilution, distillation and extraction by 1, 2-dichloroethane are carried out to obtain 1-2-(2-chlorphenyl)-1, 2-ethylene glycol, then triethylamine and mesyl chloride are added into the 1-2-(2-chlorphenyl)-1, 2-ethylene glycol for esterification reaction, and then diluted hydrochloric acid, sodium bicarbonate and drinking water are respectively used for washing, reduced pressure distillation is carried out to remove solvent, so as to obtain sticky grease, ethyl acetate is added at the temperature below 77 DEG C for dissolution, heating is carried out until refluxing, temperature reduction, crystallization, centrifugation and drying are carried out, thus obtaining yellow solid 1-(2-chlorphenyl)-1, 2-glycol dimethyl sulphonate. Then dimethyl sulphoxide and potassium hydroxide are taken as raw materials, mixed liquid of 1-imidazoly acetonitrile, carbon disulphide and dimethyl sulphoxide is dropwise added for condensation reaction, mixed liquid of dimethyl sulphoxide and 1-(2-chlorphenyl)-1, 2-glycol dimethyl sulphonate is dropwise added for ring formation reaction, and ice analysis, extraction by ethyl acetate and reduce pressure distillation are carried out, thus obtaining E / Z-alpha-[4-(2-chlorphenyl)-1, 3-dithiolane-2-subunit]-1H-imidazolyl acetonitrile. The E / Z-alpha-[4-(2-chlorphenyl)-1, 3-dithiolane-2-subunit]-1H-imidazolyl acetonitrile is separated by silicagel column and then added with active carbon, distillation is carried out to remove most solvent, hot pressing filtering, temperature reduction, crystallization, centrifugation and drying are carried out, thus obtaining almost white or white lanoconazole powder solid.
Owner:傅军
Catalyst used in process of preparing vinyl chloride from 1,2-ethylene dichloride and preparation method of catalyst
ActiveCN102247884BPreparation by hydrogen halide split-offMolecular sieve catalystsRare-earth elementPtru catalyst
The invention provides a catalyst used in the process of preparing vinyl chloride from 1,2-ethylene dichloride and a preparation method of the catalyst. The catalyst consists of 10.0 to 80.0 weight percent of zeolite and 20.0 to 90.0 weight percent of inorganic oxide substrate, wherein the zeolite is a zeolite socony mobil-5 (ZSM-5) molecular sieve which comprises a rare earth element and has a melt flow index (MFI) structure; the rare earth element is 1 to 15 weight percent based on RE2O3; RE represents the rare earth element; and the inorganic oxide substrate can be a pure oxide or a complex of a plurality of oxides. In the preparation method, the catalyst is prepared by a spray drying forming method. Compared with the conventional thermal cracking technology, the method has the advantages that: energy consumption can be greatly reduced, production cost is reduced, and the catalyst is suitable for a fluidized bed reaction device.
Owner:天津渤化化工发展有限公司
Laser-effect coating composition for PP substrate and preparation method thereof
The present invention discloses a laser-effect coating composition for a PP substrate and a preparation method thereof. The coating composition comprises the following components in percentage by weight: 5-15% of chlorinated polyolefin resin, 10-30% of phenolic resin, 1.5%-5% of amino resin, 0.01%-0.5% of auxiliaries, and 50%-80% of solvent, wherein the chlorinated polyolefin resin is at least oneof chlorinated polyethylene resin, chlorinated polypropylene resin, and ethylene chloride-propylene chloride copolymer resin. The laser-effect coating composition for the PP substrate disclosed by the present invention has the advantages of convenience of preparation and use, storage stability, good coating adaptability, moderate price and the like. In practical application, the laser-effect coating composition for the PP substrate, as a middle layer of a product, has excellent adhesion to both a bottom PP film and an upper aluminum layer.
Owner:SHANGHAI CHENGYING NEW MATERIALS +1
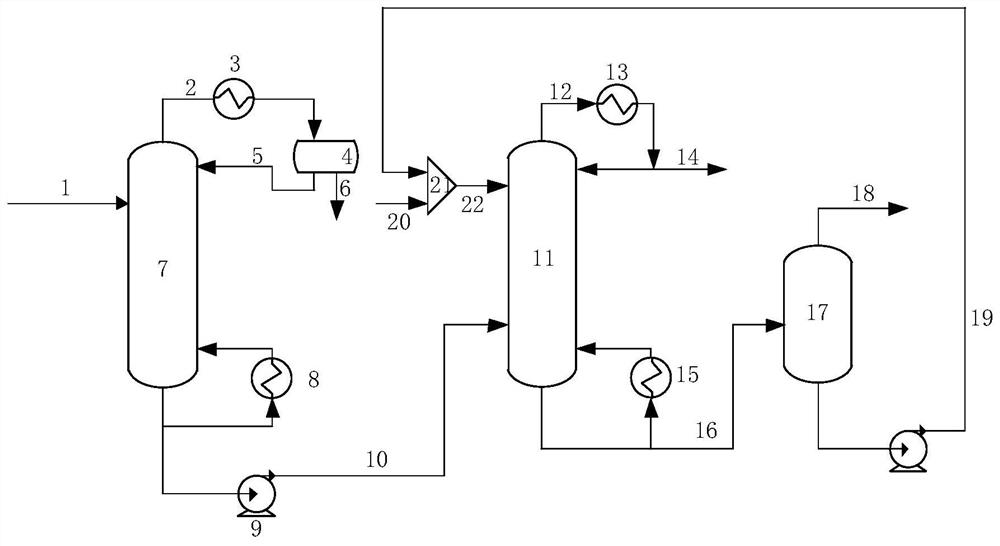
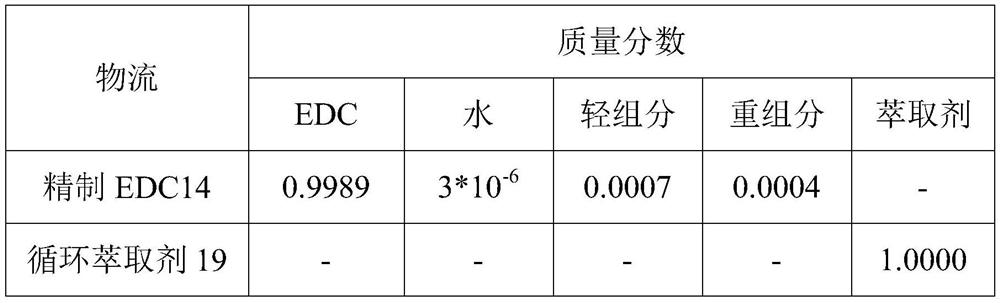
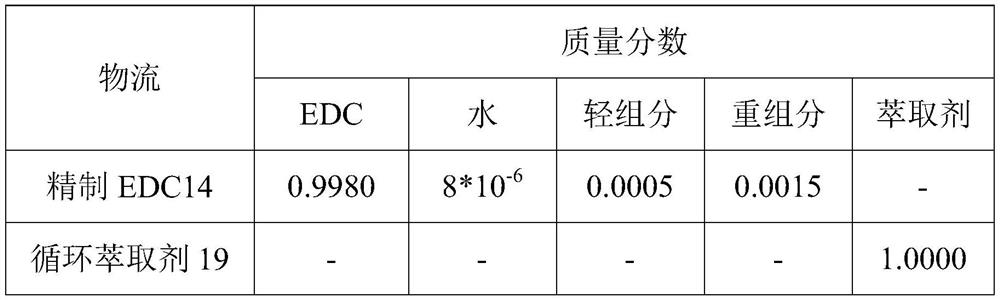
1, 2-dichloroethane purification method in vinyl chloride production process
ActiveCN111848335AIncrease relative volatilityEnergy consumption breakHalogenated hydrocarbon separation/purificationOXALIC ACID DIHYDRATEPurification methods
The invention provides a 1, 2-dichloroethane purification method in a vinyl chloride production process. The method comprises the following steps: 1) a crude EDC material enters a dehydration and light component removal tower to remove a water and light component mixture, and a mixture of EDC and heavy components is obtained at the bottom of the tower; 2) the mixture of EDC and heavy components and an extraction agent enter an extractive distillation tower for extractive distillation, refined EDC is extracted from the top of the extractive distillation tower, and a mixture of the extraction agent and the heavy components is obtained from the bottom of the tower; and 3) the mixture of the extraction agent and the heavy component enters a flash tank for separation, the heavy component is extracted from the top of the flash tank, and the extraction agent is extracted from the bottom of the flash tank. The extracting agent is a mixed extracting agent of choline chloride + oxalic acid (1: 1.5) and choline chloride + ethylene glycol (1: 4), the mass purity of the target product refined EDC is 99.60% or above, and the water content is smaller than 15 ppm. According to the invention, the traditional five-tower and three-tower process is simplified into a separation process of two towers and a flash tank, so that the equipment investment and the energy consumption are reduced.
Owner:万华化学(福建)有限公司 +1
Catalyst for preparing vinyl chloride by low-temperature dehydrochlorination of ethylene dichloride, preparation method and use
ActiveCN106391125BLow cracking temperatureExtend your lifePreparation by hydrogen halide split-offOrganic-compounds/hydrides/coordination-complexes catalystsMetal chloridePhosphonium salt
Owner:TIANJIN UNIV
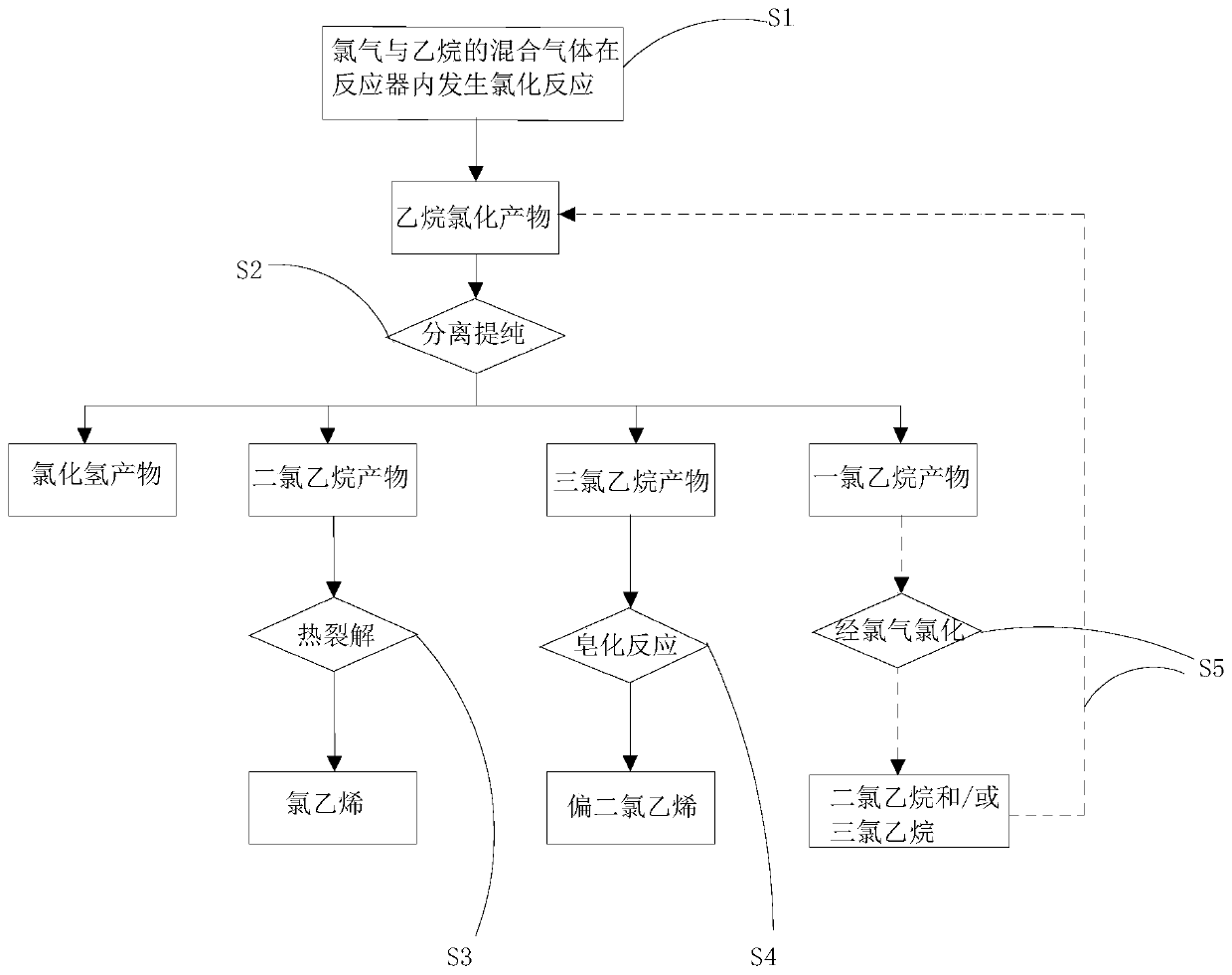
Method of producing chlorides from ethane
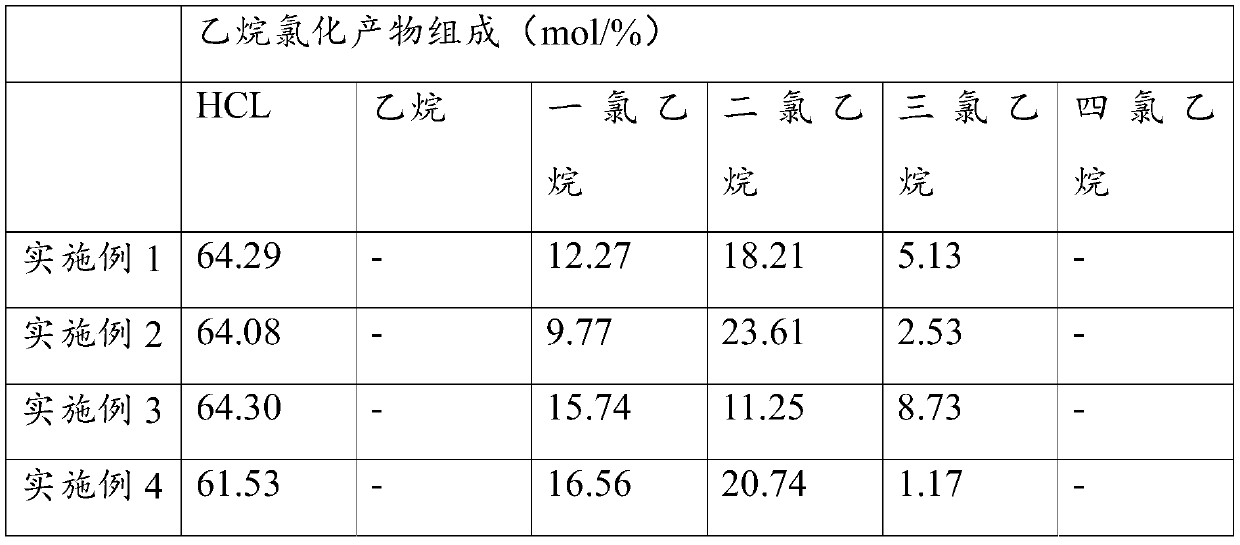
PendingCN110803975AReduce manufacturing costHigh selectivityPreparation by hydrogen halide split-offChemical synthesisWater chlorination
The invention relates to a method of producing chlorides from ethane, and relates to the field of chemical synthesis. The method comprises following steps: making mixed gas composed of chlorine gas and ethane carry out reactions in a reactor under a pressure of 0.15-0.35 MpaG to obtain ethane chlorides comprising ethane dichloride and ethane trichloride, wherein the temperature of the outlet of the reactor is 150 to 320 DEG C. The ethane chlorides are separated and purified to obtain ethane dichloride and ethane trichloride. Ethane dichloride is thermally cracked to obtain vinyl chloride. Ethane trichloride is saponified to obtain vinylidene chloride. Ethane is taken as the raw material, and chlorine is taken as the chlorinating agent to carry out chlorination reactions. Through simple separation, separated ethane dichloride is thermally cracked to obtain vinyl chloride, separated ethane trichloride is saponified to obtain vinylidene chloride, the preparation cost of vinyl chloride andvinylidene chloride is reduced, at the same time, the economic benefits and environmental benefits are obviously increased, and the method is suitable for industrial production.
Owner:杰瑞德工业设备(北京)有限公司
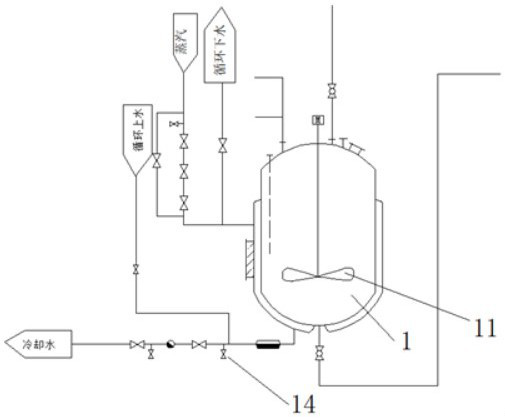
A heat exchange control device for ethyl chloride synthesis reactor
ActiveCN112710112BEasy to control temperatureDomestic cooling apparatusLighting and heating apparatusThermodynamicsCooling coil
The invention discloses a heat exchange control device for an ethyl chloride synthesis reactor. The control mechanism for controlling the type and quantity of water passing through, the heat exchange control device for the ethyl chloride synthesis reactor, by setting the control structure of the circulating water in the cooling coil group, the control mechanism can automatically control the low-pressure water and circulation of the discharged air The entry sequence of the high-pressure water used, and while controlling the sequence, it can also realize the input of individual pipes of the cooling coil group one by one, so as to better control the temperature and reaction pressure.
Owner:NINGBO JUHUA CHEM TECH CO LTD
Efficient and stable VOCs oxidative degradation pt/ceo 2 -tio 2 Preparation method of catalyst
ActiveCN111408371BLow costSimple preparation processCatalyst carriersCatalyst activation/preparationTitanium chloridePtru catalyst
The invention provides a highly efficient and stable Pt / CeO for oxidative degradation of VOCs 2 -TiO 2 The preparation method of the catalyst uses cheap cerous chloride or cerous nitrate, titanium tetrachloride, and a small amount of chloroplatinic acid as raw materials, and prepares CeO by precipitation 2 -TiO 2 Composite oxide support, Pt supported by electronless deposition method to prepare Pt / CeO 2 -TiO 2 Catalyst, CeO in catalyst 2 / TiO 2 The best molar ratio is 4:1~1:1. The preparation process of the catalyst is simple and the cost is low. The catalyst prepared by the present invention is especially suitable for catalytic combustion of organic waste gas containing chlorine-containing VOCs or non-chlorine-containing VOCs such as benzene and n-hexane, and the complete oxidation temperature of the catalyst to dichloroethane and n-hexane is only 290 ° C and At about 140°C, the catalytic activity does not decrease when they coexist, and has excellent low-temperature catalytic oxidation degradation activity and stability, and has a good application prospect.
Owner:ZHEJIANG UNIV
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008387AImprove adsorption capacityBurning at high temperature makes the organic matter completely carbonized and strong adsorptionCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of environment-friendly and chemical catalysts. The preparation method comprises the following steps: by taking attapulgite, diopside, basalt, blodite, brucite and serpentinite porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant trilaurylammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer, namely borax and potassium sulfate, catalytic activity assistant precursors, namely praseodymium(III) tris[3-(trifluoroacetyl)-D-camphor], promethium tricyclopentadienide, holmium oxalate and lutetium carbonate hydrate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound ferrous fumarate and nickel citrate and precious metal compound tris(bipyridine)ruthenium(ii)chloride hexahydrate and tetraammine dichloropalladium in a hydrothermal reactor under the action of an emulsifier 2,6-di(diethylaminomethyl)-4-nonylphenol-epichlorohydrin quaternary ammonium salt, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
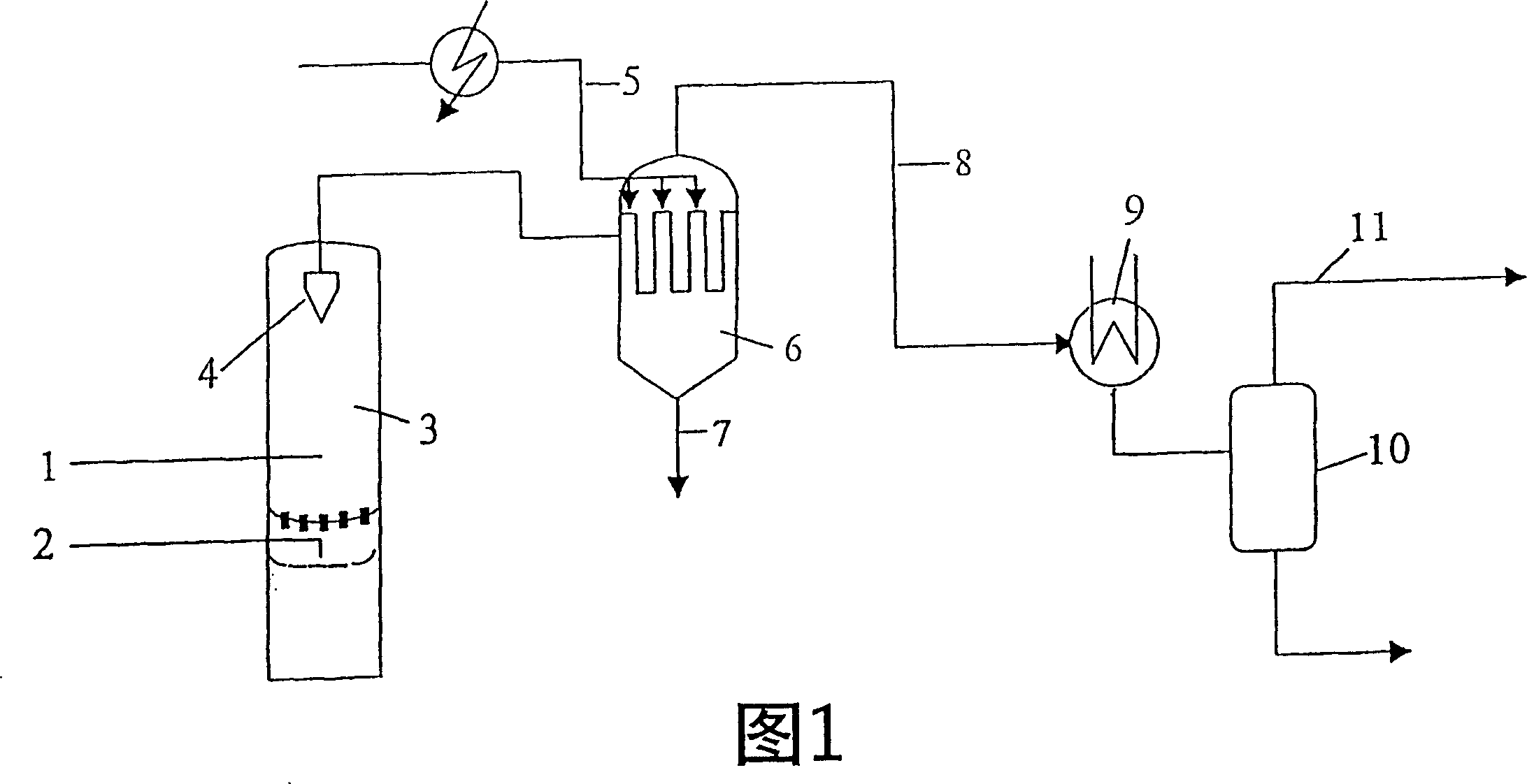
Production of 1, 2-dichloroethane
InactiveCN1308274CReduce contentImprove separation efficiencyOrganic chemistry methodsHalogenated hydrocarbon preparationFluidized bedPhysical chemistry
The invention relates to a method and a device for producing 1,2-dichloroethane by reacting ethylene with hydrogen chloride and an oxygen-containing gas in an oxychlorination reactor having a fluidised bed, in order to form a reaction gas. Said reaction gas is filtered outside the oxychlorination reactor by means of at least one filter candle.
Owner:芬诺利特技术两合公司
A kind of preparation method of 1,1-difluoroethylene
ActiveCN109180420BIncrease added valueRaw materials are cheap and easy to getPreparation by hydrogen halide split-offCatalyst activation/preparationPtru catalystReaction temperature
The invention discloses a preparation method of 1,1-difluoroethylene, comprising the following steps: a composite catalyst is loaded into a tubular reactor, and 1,1-difluoro-1-chloroethane or 1,1 -Difluoro-2-chloroethane is passed into the tubular reactor as a raw material, and gas-phase catalytic cracking reaction is carried out to obtain 1,1-difluoroethylene; the composite catalyst is that the composite catalyst is a metal Complex fluoride. The method for preparing 1,1-difluoroethylene provided by the invention has the characteristics of low reaction temperature, high product selectivity, high yield, strong catalyst reproducibility, mild preparation process conditions, simple operation and the like.
Owner:ZHEJIANG UNIV OF TECH
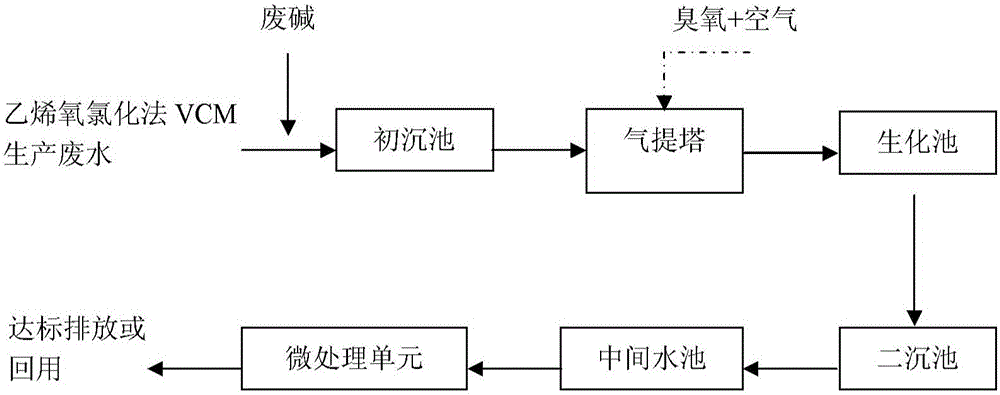
A kind of treatment method of ethylene oxychlorination process vinyl chloride production wastewater
ActiveCN104150682BEasy to handleMultistage water/sewage treatmentNature of treatment waterChemical oxygen demandSludge
The invention discloses a treatment method of vinyl chloride production wastewater by virtue of an ethylene oxychlorination process. The method comprises the following steps: performing flocculation and sedimentation action on the wastewater, and removing pollutants in the wastewater; enabling the wastewater to enter a gas stripping tower, transferring volatile chlorinated hydrocarbon in the wastewater to a gas phase from an aqueous phase, blowing off volatile chlorinated hydrocarbon from the tower top, and introducing ozone to perform catalytic oxidation treatment on the pollutants in the wastewater under the action of a catalyst at the same time; conveying the wastewater into a biochemical pool, and removing organic pollutants by virtue of biochemical treatment; enabling the wastewater to enter a secondary sedimentation tank, performing solid-liquid separation to remove suspended sludge, and then entering a middle water tank; performing deep treatment on the wastewater in a micro-treatment unit, further removing the organic pollutants which are not easy to degrade in water, and finally discharging effluent on standard or recycling the effluent. The treatment method disclosed by the invention is suitable for the vinyl chloride production wastewater by virtue of the ethylene oxychlorination process and a balanced oxychlorination process, does not need to introduce other wastewater as dilution water, and can ensure that the effluent COD (chemical oxygen demand) is less than 50mg / L.
Owner:李开明
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com