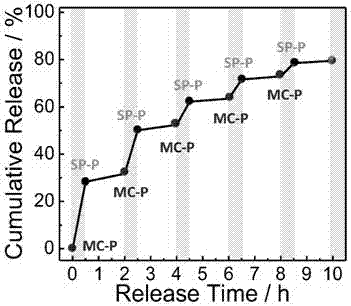
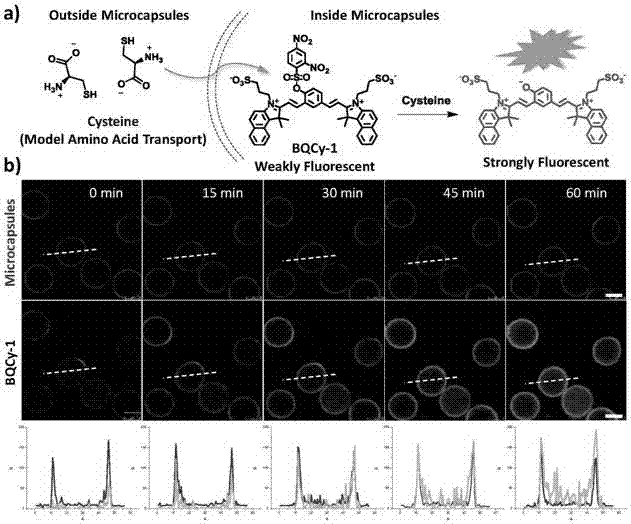
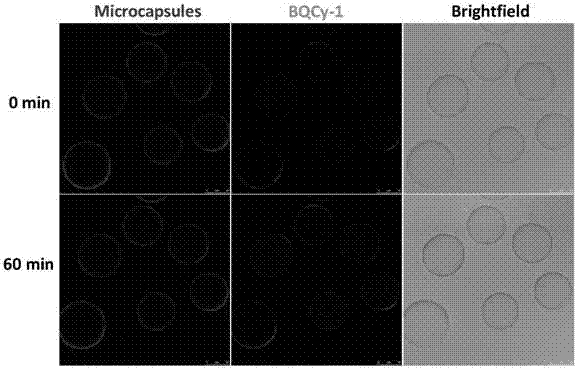
Photochromic monomer, its preparation method, amphiphilic polymer, its preparation method and vesicle and its application
An amphoteric polymer, photochromic technology, applied in the field of cells, can solve the problems of irreversibility and regulation of photolysis, and achieve the effect of controllable regulation and improvement of permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The present application also provides a preparation method of the photochromic monomer, comprising the following steps:
[0099] reacting the compound having the structure of formula (III) and the compound having the structure of formula (IV) in a solvent under the action of a catalyst to obtain a photochromic monomer;
[0100]
[0101] Among them, R 1 is H or MeO;
[0102] R 2 for NO 2 or CN;
[0103] R 3 for H or CH 3 ;
[0104] n is 1-11.
[0105] The photochromic monomer described in the present application is obtained by reacting the hydroxyl group in the compound of formula (III) with the isocyanate group of the compound of formula (IV).
[0106] In the above process of preparing the photochromic monomer, the solvent is preferably dichloromethane, and the catalyst is preferably dibutyltin dilaurate.
[0107] According to the present invention, after synthesizing the photochromic monomer, use it as the hydrophobic segment of the polymer, and polymerize ...
Embodiment 1
[0167] Dissolve 1 molar equivalent of the hydroxyethyl-functionalized photochromic molecular precursor represented by formula (VII) in 30 mL of dry dichloromethane, add 0.5% DBTL as a catalyst, Add 1.5 molar equivalents of isocyanoethyl methacrylate represented by formula (Ⅷ), stir and react for 7 to 24 hours, wash with saturated brine twice after the reaction, add anhydrous MgSO 4 Dry, remove the solvent by rotary evaporation under reduced pressure, separate and purify by column chromatography using dichloromethane-ethyl acetate as eluent to obtain the corresponding product, which is denoted as M1 monomer, and its yield is 75-92%. The structure of the M1 monomer was characterized by H NMR, C NMR and high-resolution mass spectrometry, and the results showed that Figure 1~3 shown.
[0168]
[0169] In the above formula, R 1 for H, R 2 for NO 2 , R 3 is H, n is 1.
Embodiment 2
[0171] 1.2g of M1 monomer, 0.3g of PEG-CTA chain transfer agent shown in formula (IX) and 5.0mg of AIBN initiator were dissolved in 4mL of dioxane, then added to a sealed tube containing a magnetic stirrer, fully removed Seal the tube after gas, place it in an oil bath at 70°C, stop the reaction after 7-12 hours of polymerization, and precipitate in anhydrous ether, precipitate three times, and dry in a vacuum oven to obtain polymer P1. Figure 4 NMR characterization of polymer P1.
[0172]
[0173] Among them, R 1 for H, R 2 for NO 2 , R 3 is H, x is 19, n is 1, and m is 45.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com