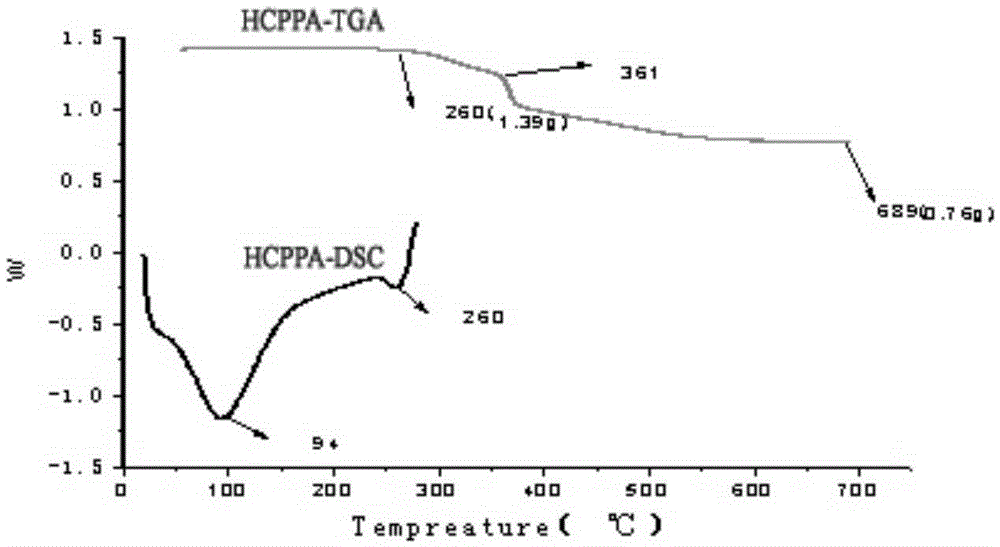
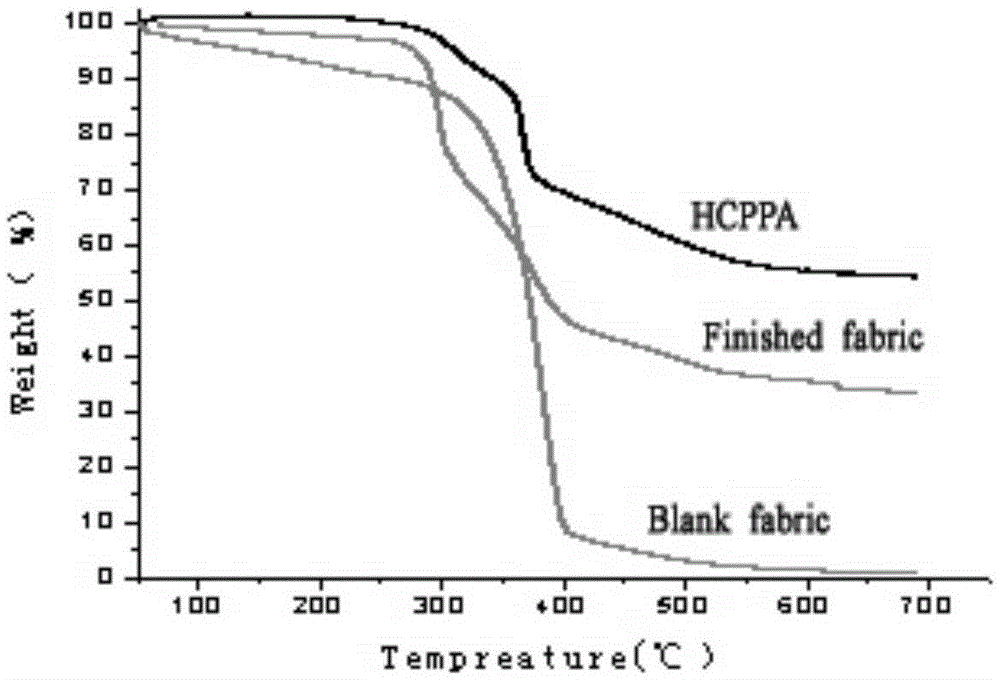
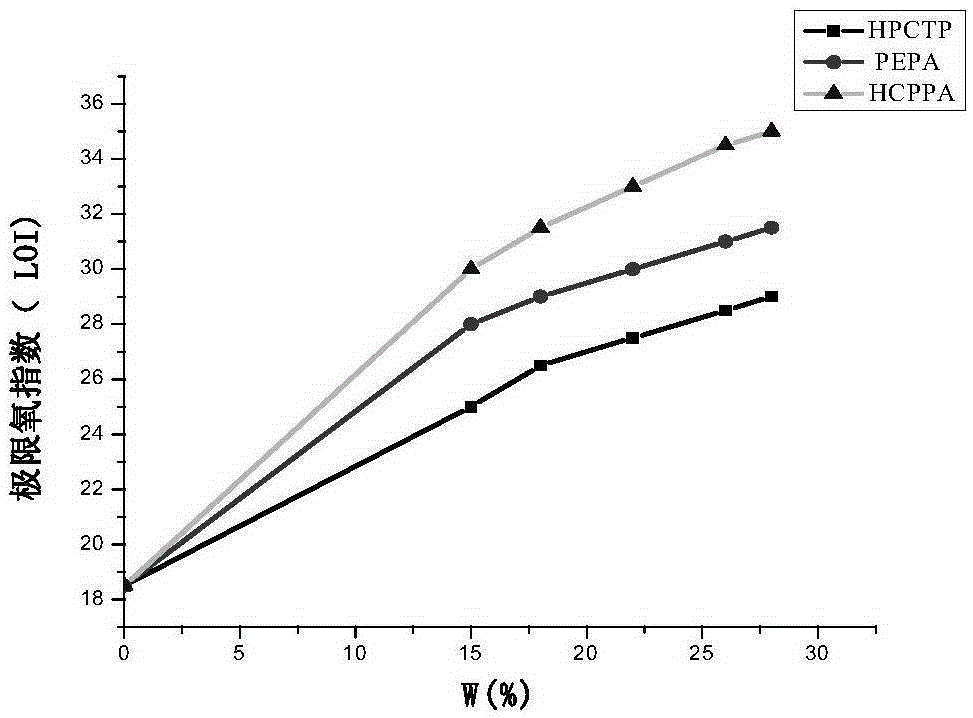
N-P flameresistant material and preparation method thereof and application in textiles
A flame retardant material and reaction technology, which is applied in the field of flame retardant material preparation, can solve the problems of low carbon formation rate and poor thermal stability, and achieve the effects of high carbon formation rate, controllable reaction and high limiting oxygen index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of Hexachlorocyclotriphosphazene (HCPP)
[0048]In a 250mL three-neck flask with a constant pressure dropping funnel and a magnetic stirring device, add 16.0g of finely ground ammonium chloride and 120mL of chlorobenzene in sequence; then weigh 34.0g of phosphorus pentachloride in a 100mL beaker, and add 80mL Chlorobenzene, heating, stirring evenly, after phosphorus pentachloride is completely dissolved in chlorobenzene, quickly transfer to a constant pressure funnel, and take insulation measures to prevent phosphorus pentachloride from chlorobenzene solution precipitation. Turn on the heating device under the protection of nitrogen, and when the temperature of the solution in the three-necked flask reaches 120-130°C, start to add the chlorobenzene solution of phosphorus pentachloride dropwise at a uniform speed, and the dropping time should be controlled at about 2.5 hours. , reacted for about 2-3h, stopped heating, cooled, suction filtered, and rotary evapor...
Embodiment 2
[0050] Synthesis of 1-Oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2.2]octane (PEPA)
[0051] Add 44.8g of pentaerythritol and 130mL of 1,4-dioxane into a three-necked flask with a constant pressure dropping funnel and a tail gas receiving device, and then measure 33.6mL of POCl 3 Add 40mL of 1,4 dioxane to the constant pressure funnel and heat it. When the temperature reaches 85°C, start to add phosphorus oxychloride dropwise. It takes about 2.5 hours for the dropwise addition to be completed. React for 2-3h, then heat up to reflux state, react for about 2-4h, filter with suction, recrystallize to get white needle-like crystal PEPA, the yield is 88%, M.P.209.5-212.3°C, IR (KBr, cm-1 ): 869, 987, 1226, 1299, 2959, 3391; 1 HNMR (400MHZ, CDCl3, TMS, δppm): 5.09 (d, 1H), 4.58 (m, 6H), 3.28 (d, 1H).
Embodiment 3
[0053] Synthesis of N-P Flame Retardant Material (HCPPA)
[0054] Weigh 10.8g of PEPA and an appropriate amount of NaH in a three-necked flask, measure 60mL of acetonitrile solution into the three-necked flask, and add an equimolar amount of pyridine to the three-necked flask, heat to 60°C, and mechanically stir Pour 3.3g of hexachlorocyclotriphosphazene into a three-necked flask, react at reflux temperature for 1-3 hours, wash twice with water, then wash once with absolute ethanol, and dry in a constant temperature oven at 60-80°C. A white powdery solid HCPPA was obtained with a yield of 66.2%. M.P. 265-268°C. Its infrared spectrum is as Figure 4 As shown, the H NMR spectrum is shown as Figure 5 Shown; IR(KBr,cm -1 ): 853, 1033, 1191, 1261, 1317, 1480, 2971, 2911; 1 HNMR (400MHZ, DMSO, TMS, δppm): 3.42 (d, 6H), 4.65 (m, 36H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com