Curcumin-containing polymeric micelle drug delivery system and preparation method and application thereof
A drug-carrying system, curcumin technology, applied in antitumor drugs, drug combinations, ketone active ingredients, etc., can solve problems such as failure, drug concentration is difficult to achieve effective therapeutic dose, etc., to improve targeting, good biological Compatibility and biodegradability, effects of strong interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
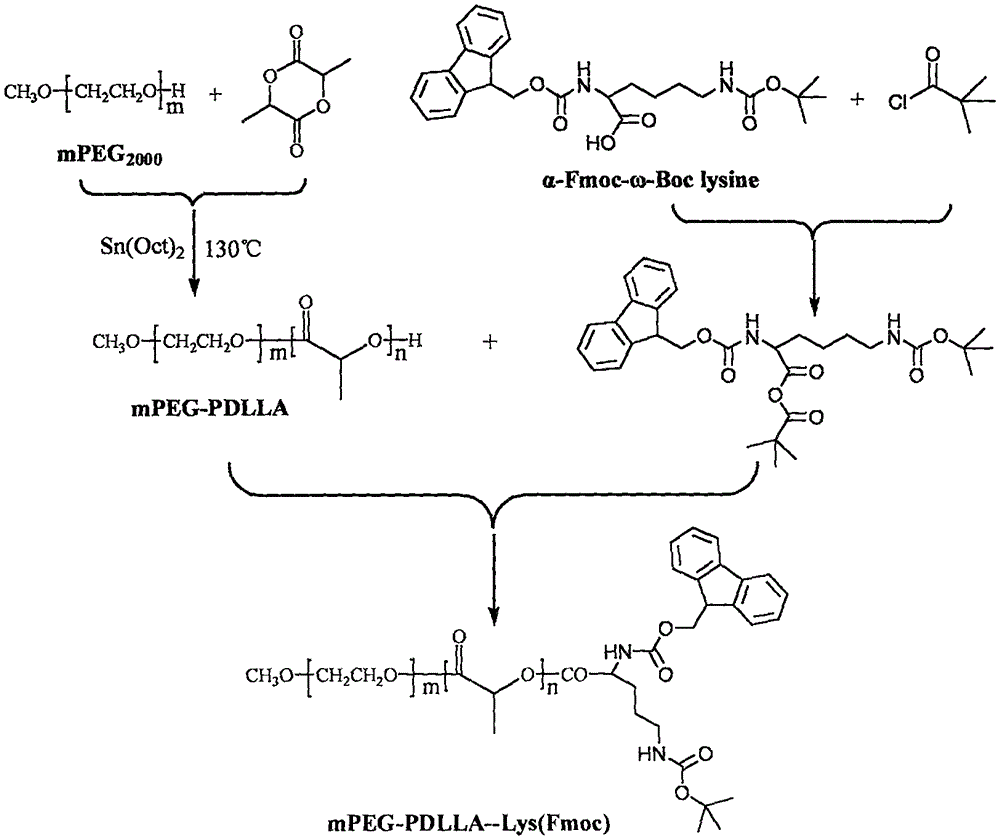
[0061] Preparation of Methoxy Polyethylene Glycol-Polylactide Block Copolymer
[0062] 1) mPEG 2000 -PLA 1300 Synthesis
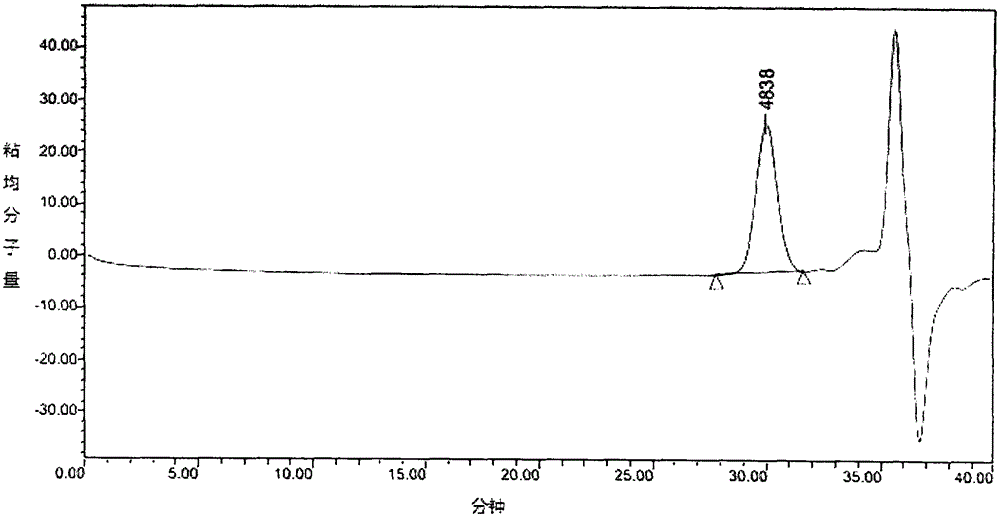
[0063] 10g of methoxy polyethylene glycol 2000 was added to the polymerization bottle, heated to 130°C and stirred and vacuumed for 1h, after cooling to room temperature, 11g of D, L-lactide and 5.5mg of stannous octoate were added. The reactants were polymerized at 130°C for 15 hours, and the product was dissolved in dichloromethane, precipitated with ice ether and filtered to obtain the white solid as mPEG. 2000 -PLA 1300 For block copolymers, the molecular weight of the polymer calculated by NMR is 3300, and the molecular weight and molecular weight distribution coefficient of the polymer determined by Gel Permeation Chromatography (GPC) are 4596 and 1.06, respectively. (Such as figure 1 and figure 1 Related data table 1).
[0064] Table 1 Generalized relative peak
[0065]
[0066] 2) Capping reaction
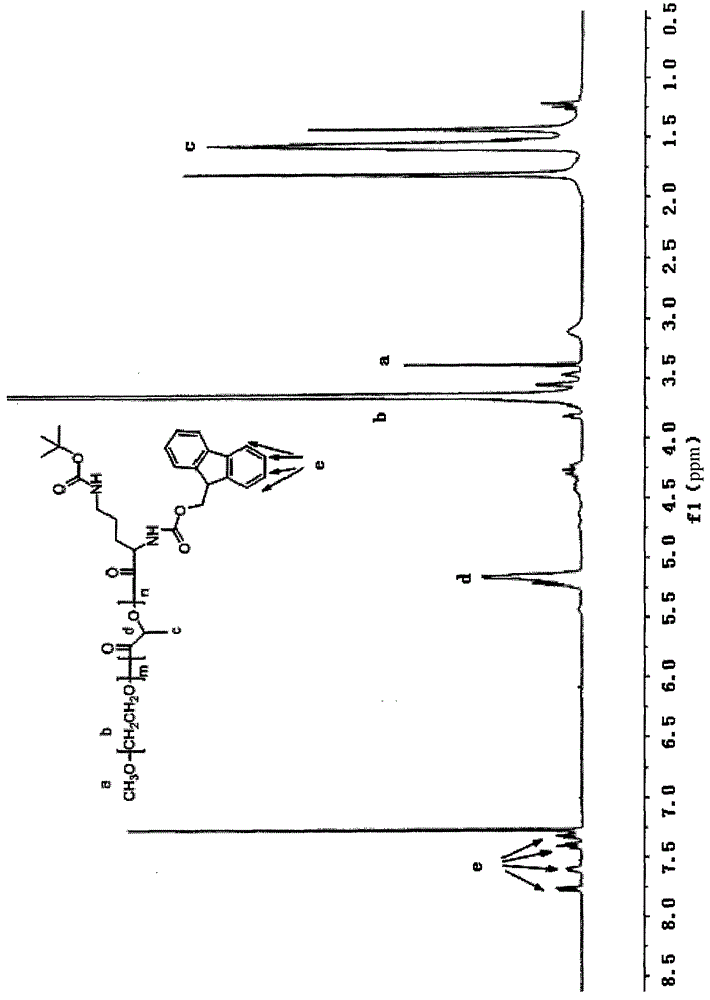
[0067] 11.7g of 6-Fluorenylmethoxycarbonylamino-2-tert-but...
Embodiment 2
[0070] 1) mPEG 2000 -PLA 1000 Synthesis
[0071] 10g of methoxy polyethylene glycol 2000 was added to the polymerization bottle, heated to 130°C, stirred and vacuumed for 1h, after cooling to room temperature, 7g of D, L-lactide and 3mg of stannous octoate were added, and the polymerization bottle was sealed in a vacuum. After polymerizing at 130℃ for 15h, the product was dissolved in dichloromethane, precipitated with ice ether and filtered to obtain the white solid as mPEG 2000 -PLA 1000 For block copolymer, the molecular weight of the polymer calculated by NMR is 3000, and the molecular weight and molecular weight distribution coefficient of the polymer determined by GPC are 3943 and 1.05 respectively.
[0072] 2) Capping reaction
[0073] 11.7g 6-Fluorenylmethoxycarbonylamino-2-tert-butoxycarbonylaminocaproic acid was dissolved in 50mL of anhydrous tetrahydrofuran and then 3-10mL of triethylamine was added. After cooling to -10℃, 2-5mL of pivaloyl chloride was added, and it immed...
Embodiment 3
[0076] 1) mPEG 2000 -PLA 1500 Synthesis
[0077] 10g of methoxy polyethylene glycol 2000 was added to the polymerization flask, heated to 130°C, stirred and vacuumed for 1h, after cooling to room temperature, added 13g of D, L-lactide, 6mg of stannous octoate, and sealed the polymerization flask in a vacuum. After polymerizing at 130℃ for 15h, the product was dissolved in dichloromethane, precipitated with ice ether and filtered to obtain the white solid as mPEG 2000 -PLA 1300 For block copolymers, the molecular weight of the polymer calculated by NMR is 3500, and the molecular weight and molecular weight distribution coefficient of the polymer determined by GPC are 4746 and 1.06 respectively.
[0078] 2) Capping reaction
[0079] 11.7g of 6-Fluorenylmethoxycarbonylamino-2-tert-butoxycarbonylaminocaproic acid was dissolved in 50mL of anhydrous tetrahydrofuran and then 3.5mL of triethylamine was added. After cooling to -10°C, pivaloyl chloride (3.05mL) was added. White precipitate imm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com