Docetaxel injection and preparation method thereof
A docetaxel, injection technology, applied in pharmaceutical formulations, oil/fat/wax inactive ingredients, drug combinations, etc., can solve problems such as interference with P-glycoprotein expression, temperature sensitivity, patient death, etc., and achieve stability Good performance, stable active ingredient content, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
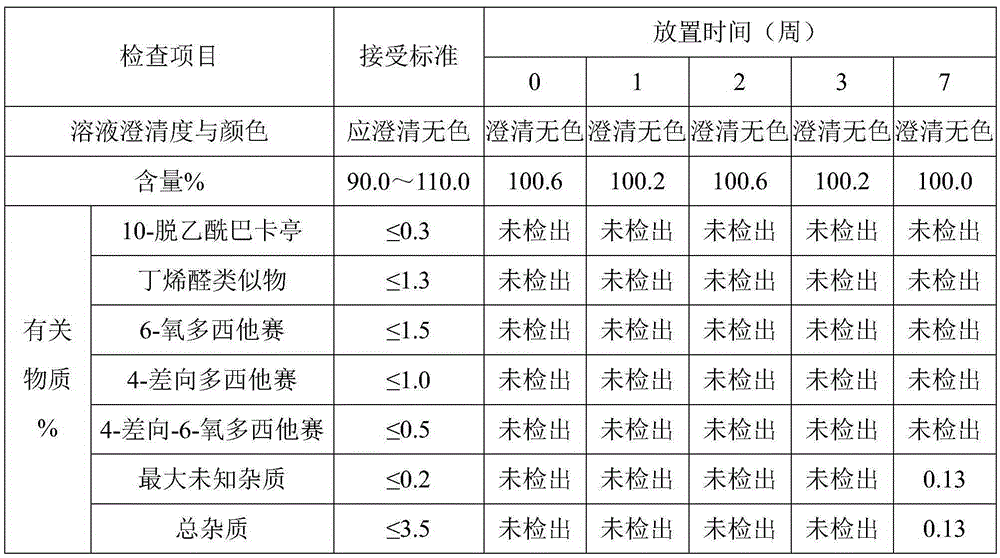
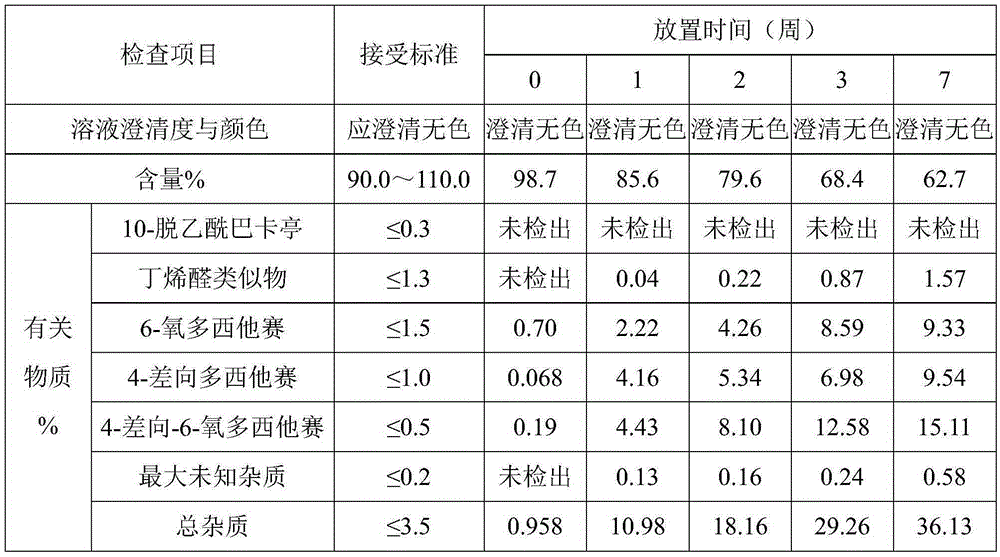
Examples
Embodiment 1
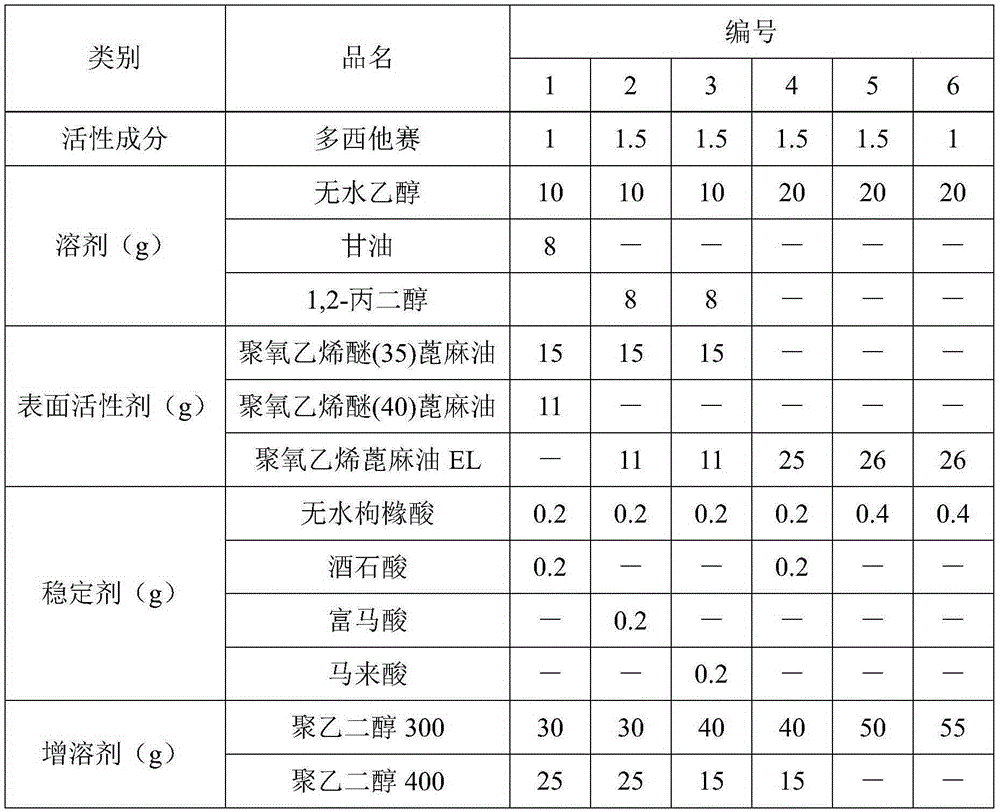
[0020] Preparation of docetaxel injection: mix 10 g of absolute ethanol and 8 g of glycerin evenly, add 0.2 g of anhydrous citric acid and 0.2 g of tartaric acid to completely dissolve, and stir well; add 1 g of docetaxel, and stir until completely dissolved; add 15g polyoxyethylene ether (35) castor oil and 11g polyoxyethylene ether (40) castor oil, stir and mix well; add 30g polyethylene glycol 300 and 25g polyethylene glycol 400, stir and mix well, you can get Dorsey Taxal injection.
Embodiment 2
[0022] Preparation of docetaxel injection: mix 10g of absolute ethanol and 8g of 1,2-propanediol evenly, add 0.2g of anhydrous citric acid and 0.2g of fumaric acid to dissolve completely, stir well; add 1.5g of docetaxel , stir until completely dissolved; add 15g polyoxyethylene ether (35) castor oil and 11g polyoxyethylene castor oil EL, stir and mix evenly; add 30g polyethylene glycol 300 and 25g polyethylene glycol 400, stir and mix evenly, namely Docetaxel injection is available.
Embodiment 3
[0024] Preparation of docetaxel injection: mix 10 g of absolute ethanol and 8 g of 1,2-propanediol evenly, add 0.2 g of anhydrous citric acid and 0.2 g of maleic acid to dissolve completely, and stir well; add 1.5 g of docetaxel , stir until completely dissolved; add 15g polyoxyethylene ether (35) castor oil and 11g polyoxyethylene castor oil EL, stir and mix evenly; add 40g polyethylene glycol 300 and 15g polyethylene glycol 400, stir and mix evenly, namely Docetaxel injection is available.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com