Melittin liposome nanometer preparation, and preparation method and applications thereof
A nano-preparation, melittin technology, applied in the direction of liposome delivery, anti-inflammatory agents, anti-fungal agents, etc., can solve the problems of toxicity, low bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation and characterization of embodiment 1 melittin-polyglutamic acid liposome nano-preparation
[0145] Preparation:
[0146] (1) Dissolve melittin and polyglutamic acid (PGA50-100KD, 500KD, 700KD) of different molecular weights in deionized water, so that the final concentration of the aqueous solution is 0.5mg / mL; add melittin solution to polygu Amino acid solution, vortexed for 10s, and incubated at room temperature for 30min to obtain melittin-polyglutamic acid nanoparticles with a mass ratio of melittin to polyglutamic acid of 2:1; using a 100KD ultrafiltration tube, Free melittin was removed by filtration, and the nanoparticles were redispersed in deionized water.
[0147] (2) DOTAP and DOPE lipids were dissolved in chloroform, and liposomes were prepared by film dispersion method, and 1 mg / mL blank cationic liposomes were obtained after hydration with deionized water. In the vortex state, according to the mass ratio of blank liposomes and melittin-polygl...
Embodiment 2
[0153] Embodiment 2 melittin-polyglutamic acid liposome nano preparation stability research
[0154] Preparation:
[0155] The melittin-polyglutamic acid (700KD) nanoparticles prepared in Example 1 were redispersed in deionized water and 4-hydroxyethylpiperazineethanesulfonic acid buffer (HEPES), prepared in Example 1 Good melittin-polyglutamic acid liposome nano-preparations were re-dispersed in deionized water, 4-hydroxyethylpiperazineethanesulfonic acid buffer solution (HEPES), 5% glucose injection and normal saline respectively. At different time points, the particle size, particle size polydispersity index (PDI) and zeta potential were measured using a dynamic light scattering nanometer particle size analyzer (DLS) to investigate its stability. After dialysis, HPLC was used to detect melittin in the dialysate to analyze the melittin leakage of the melittin-polyglutamic acid liposome nano-preparation.
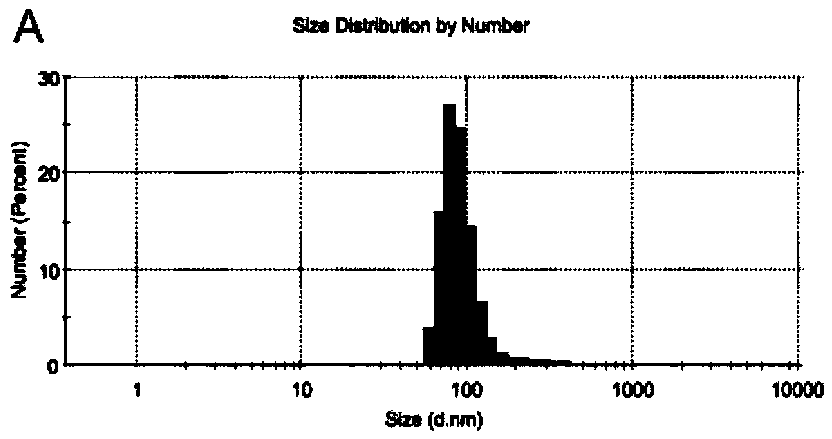
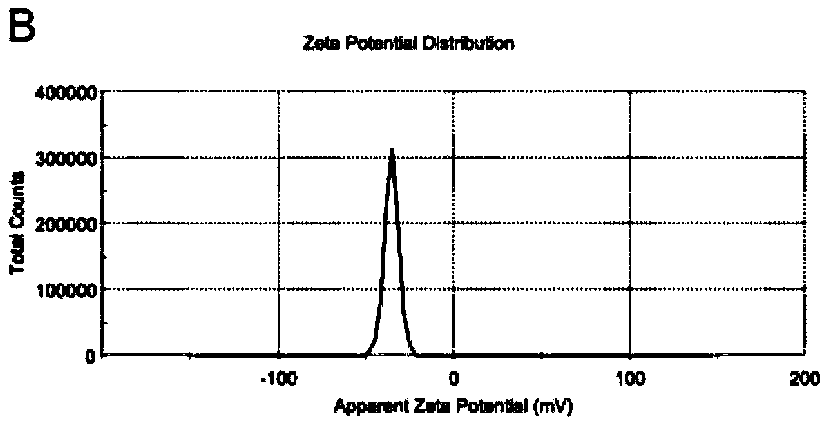
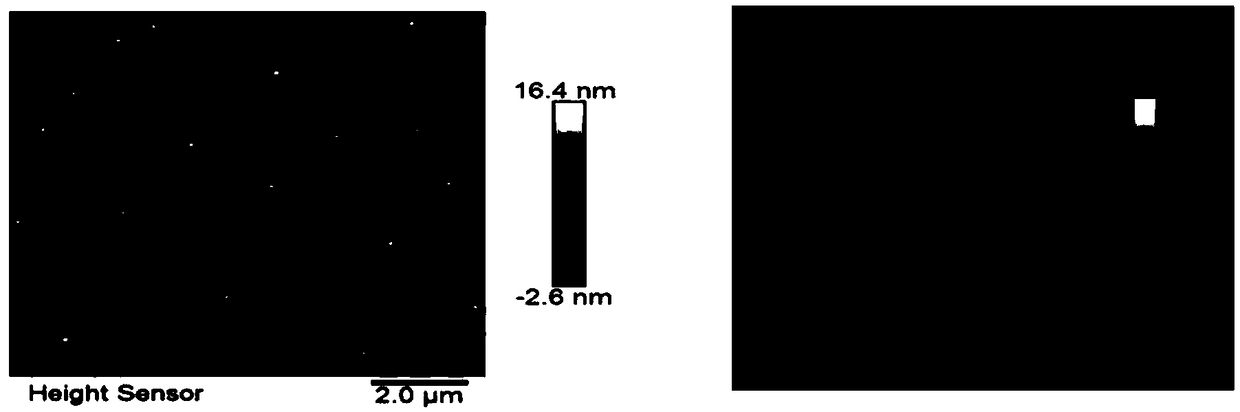
[0156] Among them, such as Figure 5A , the particle size of melitt...
Embodiment 3
[0158] The hemolytic detection of embodiment 3 melittin-polyglutamic acid liposome nano-preparation
[0159]Blood was taken from the eyes of the mice and anticoagulated with sodium heparin. After counting using a hemocytometer, the blood was divided into EP tubes so that each tube contained 5x10^6 blood cells; 2000rpm, centrifuged for 5min to remove the supernatant, and an equal volume of normal saline was added. The melittin-polyglutamic acid nanoparticles obtained in Example 1 and the melittin-polyglutamic acid liposome nano-preparation and free melittin medicine are dispersed in HEPES to obtain different concentrations (by contained melittin amount ) solution and added to the EP tube, incubated at 37°C for 6 hours.
[0160] Afterwards, centrifuge again, collect the supernatant and put it into a 96-well plate, and set up three groups of parallel samples; use a microplate reader for detection, and the detection wavelength is 525nm.
[0161] Hemolysis rate (%) = [(As-Ab) / (Ac-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrated particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com