High-performance liquid chromatography-triple quadrupole mass spectrometry combination method for determining paclitaxel or paclitaxel
A technology of high-performance liquid chromatography and triple quadrupole, which is applied in the field of pharmaceutical analysis and can solve problems such as mass spectrometer contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
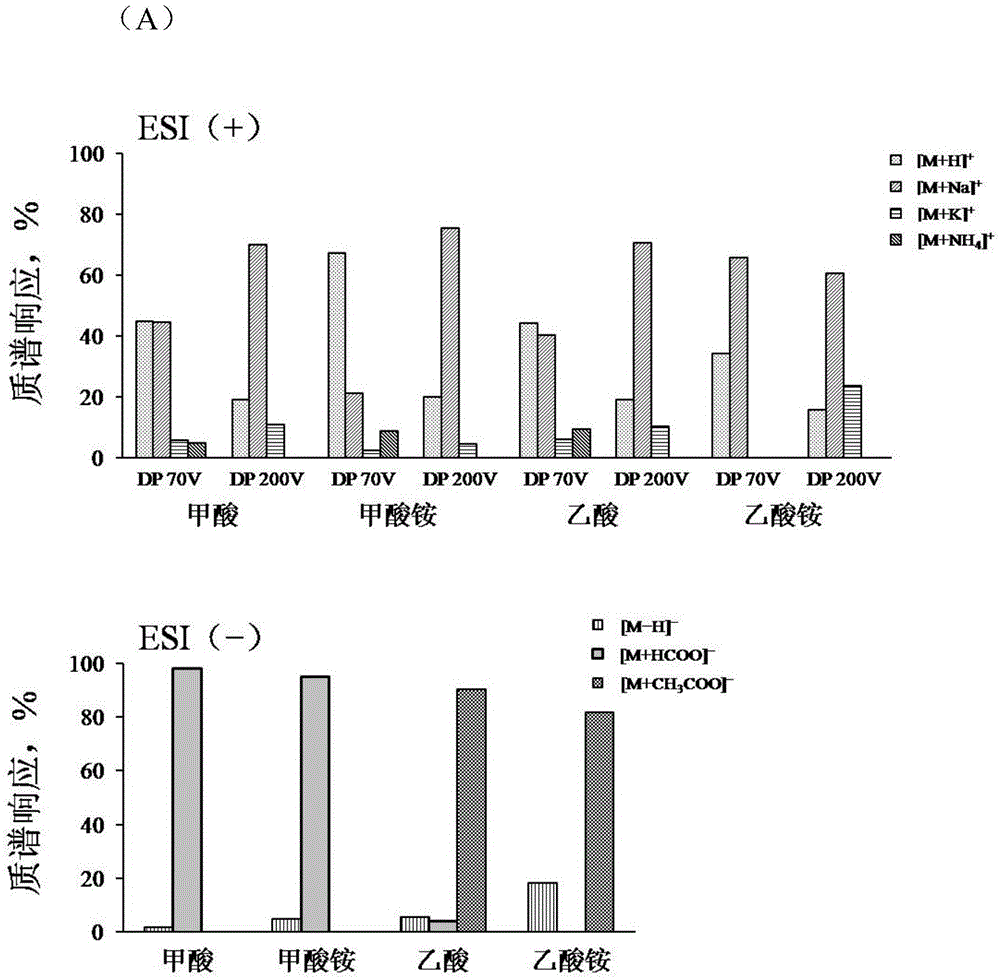
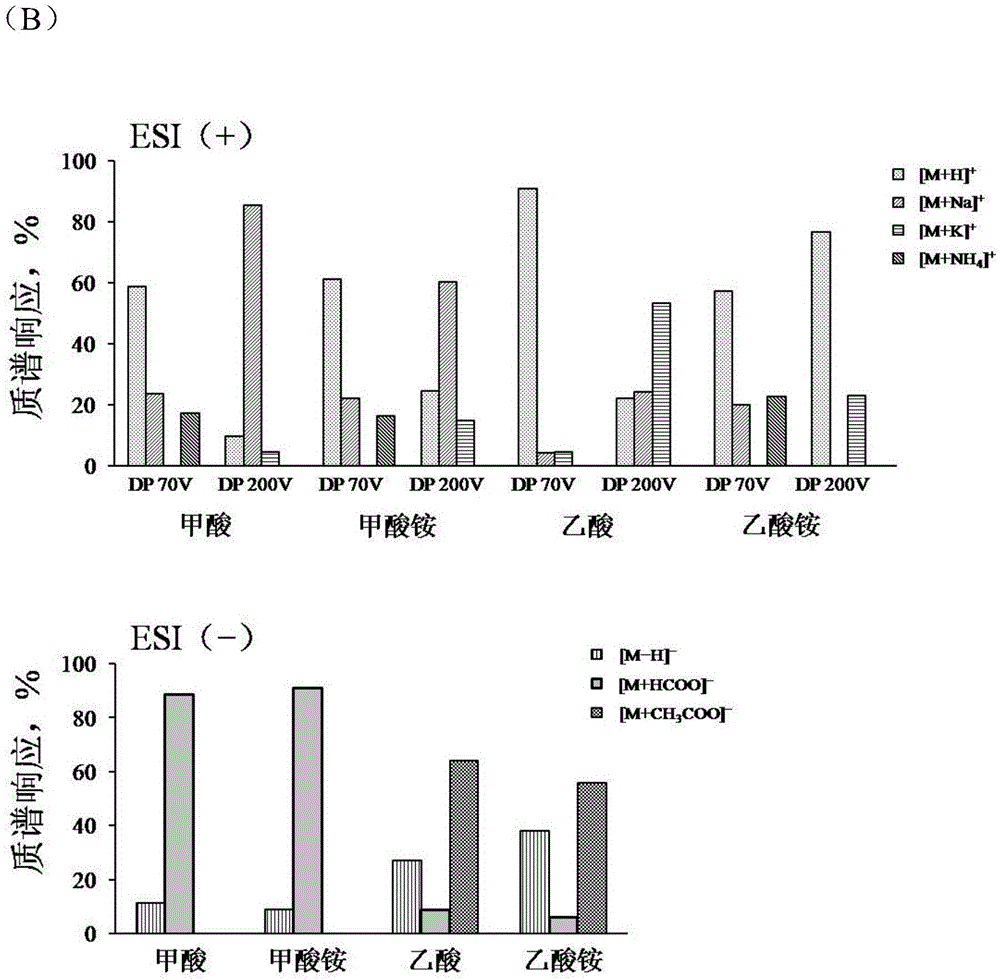
[0082] Example 1 Mass Spectrometry Ionization Forms of Docetaxel and Paclitaxel in Different Ionization Modes
[0083] experimental method:
[0084] 1. Concentration of paclitaxel or docetaxel reference substance solution: 50 μg / mL, mixed with the liquid phase mobile phase through the T-shaped tee by needle pump injection, and then enters the mass spectrometer detector.
[0085] 2. Liquid mobile phase: solvent A: aqueous solution; solvent B: acetonitrile. The electrolytes and concentrations in solvent A are: (1) 1‰ formic acid; (2) 1‰ acetic acid; (3) 10mM ammonium formate; (4) 10mM ammonium acetate.
[0086] 3. Liquid-mass spectrometry analysis conditions: Chromatographic column: Agilent EclipseplusC 18 Column (2.1×50mm, 5μm); column temperature: 40°C; elution conditions: isocratic elution with 46.5% solvent B; flow rate: 0.40mL / min; mass spectrometry detection using ESI (+) or ESI (-) ionization mode .
[0087] 4. Needle pump speed: 10μL / min.
[0088] 5. Result represen...
Embodiment 2
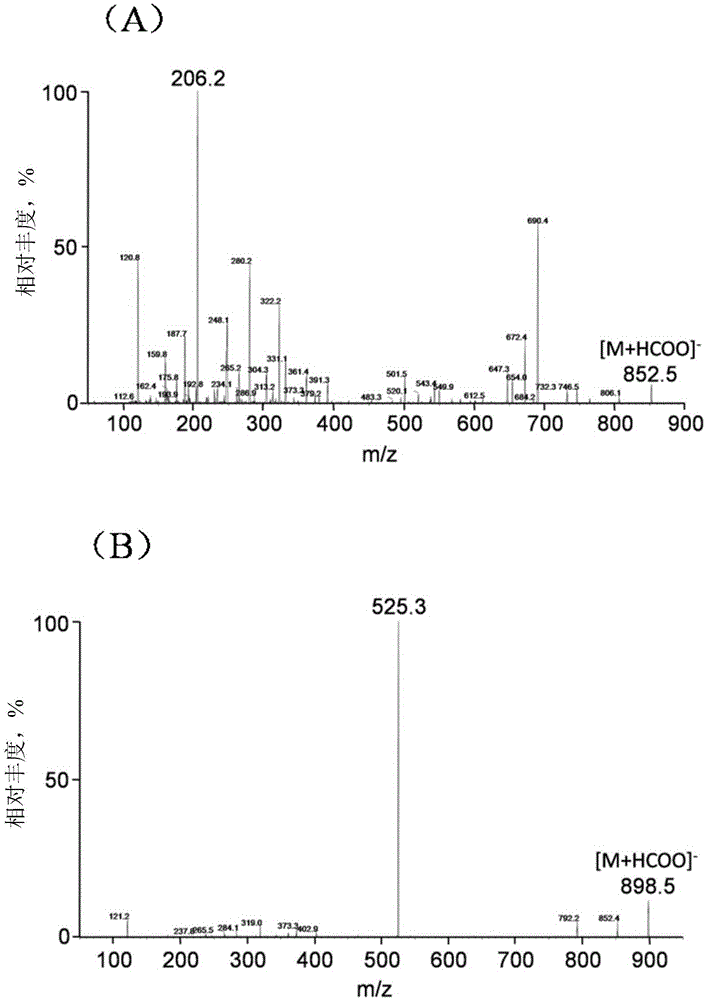
[0093] Under embodiment 2ESI (-) ionization mode, the optimization of ammonium formate concentration in the liquid phase mobile phase
[0094] experimental method:
[0095] 1. Liquid-mass spectrometry analysis conditions: Chromatographic column: Agilent EclipseplusC 18 Column (2.1×50mm, 5μm); column temperature: 40°C. Liquid mobile phase: Solvent A: H 2 O or aqueous ammonium formate; solvent B: acetonitrile. Elution conditions: isocratic elution with 46.5% solvent B. Flow rate: 0.40mL / min. Injection volume: 10 μL. The mass spectrometry adopts ESI(-) ionization mode. Under the collision energy of -51V in the MRM detection mode, the m / z852→206 ion pair of docetaxel is detected; under the collision energy of -24V, the m / z898→ of paclitaxel is detected. 525 ion pairs, mass spectrometry parameters are shown in Table 3, docetaxel and paclitaxel in ESI(-) figure 2 .
[0096] Table 3: Mass Spectrometry Working Parameters for Quantitative Analysis of Docetaxel and Paclitaxel i...
Embodiment 3
[0102] Under embodiment 3APCI (-) ionization mode, the optimization of ammonium formate concentration in the liquid phase mobile phase
[0103] experimental method:
[0104] 1. Liquid-mass spectrometry analysis conditions: Chromatographic column: Agilent EclipseplusC 18 Column (2.1×50mmID, 5μm); column temperature: 45°C; mobile phase: solvent A: H 2 O or aqueous ammonium formate; solvent B: methanol. Gradient elution conditions: elution with 20% solvent B in 0~0.1min, increasing solvent B from 20% to 60% at a constant speed in 0.1~0.5min, increasing solvent B from 60% to 90% at a constant speed in 0.51~0.8min, 0.81~1.5 Min was eluted with 90% solvent B, 1.51-1.6 min, solvent B decreased from 90% to 20% at a constant speed, and 1.61-3.5 min was 20% solvent B for column equilibration; flow rate: 0.80mL / min; injection volume: 10μL. Mass spectrometry uses APCI(-) ion source, under the collision energy of -35V in the MRM detection mode, the m / z853→280 ion pair of docetaxel is de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com