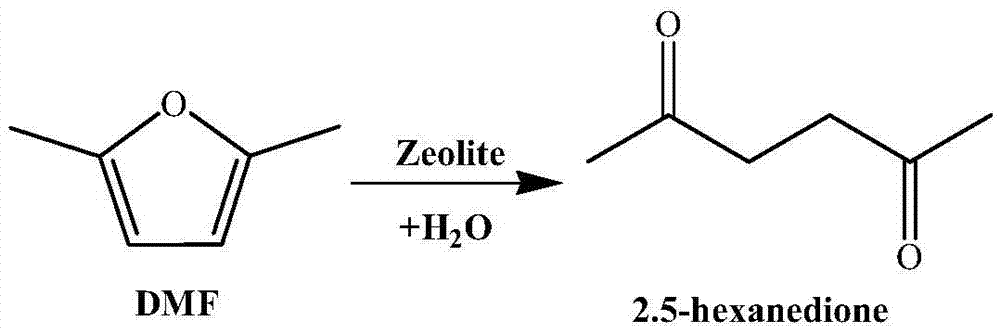
Method for preparing 2,5-hexanedione under catalysis of solid acid
A solid acid catalysis, solid acid catalyst technology, applied in the preparation of heterocyclic compounds, organic chemistry, etc., can solve the problems of difficult separation of reaction products, environmental pollution, etc., to achieve the effect of suppressing side reactions, reducing the difficulty of separation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
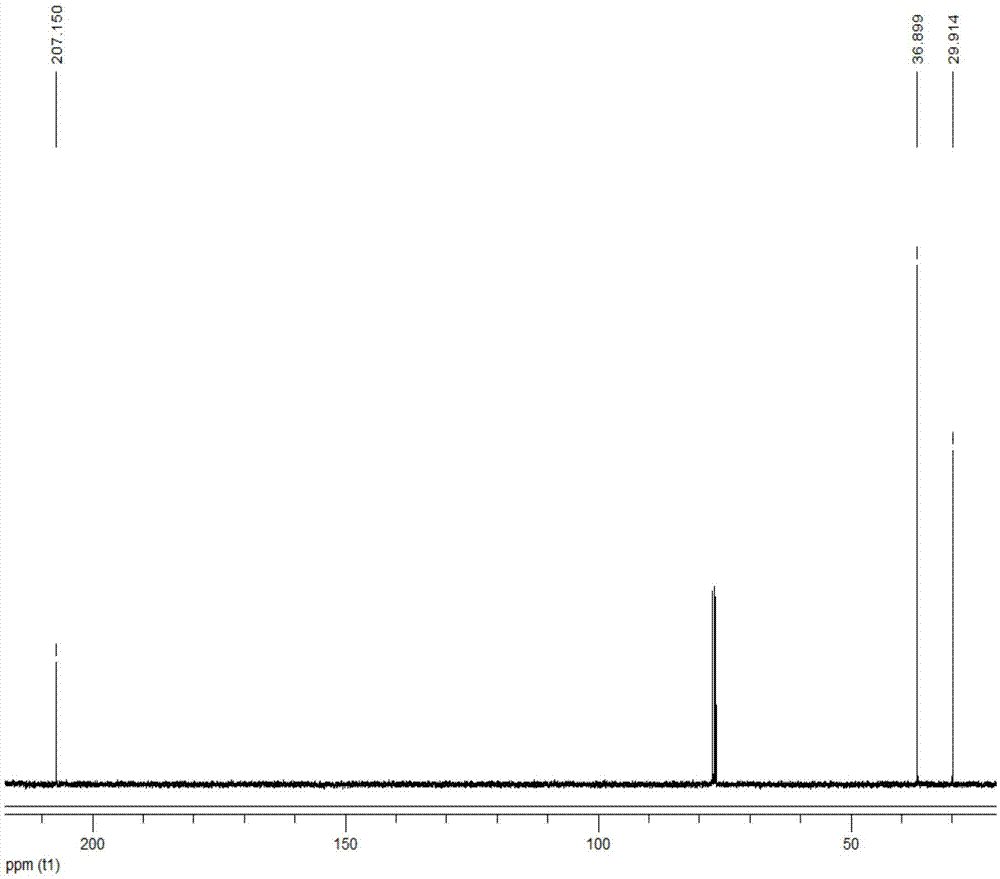
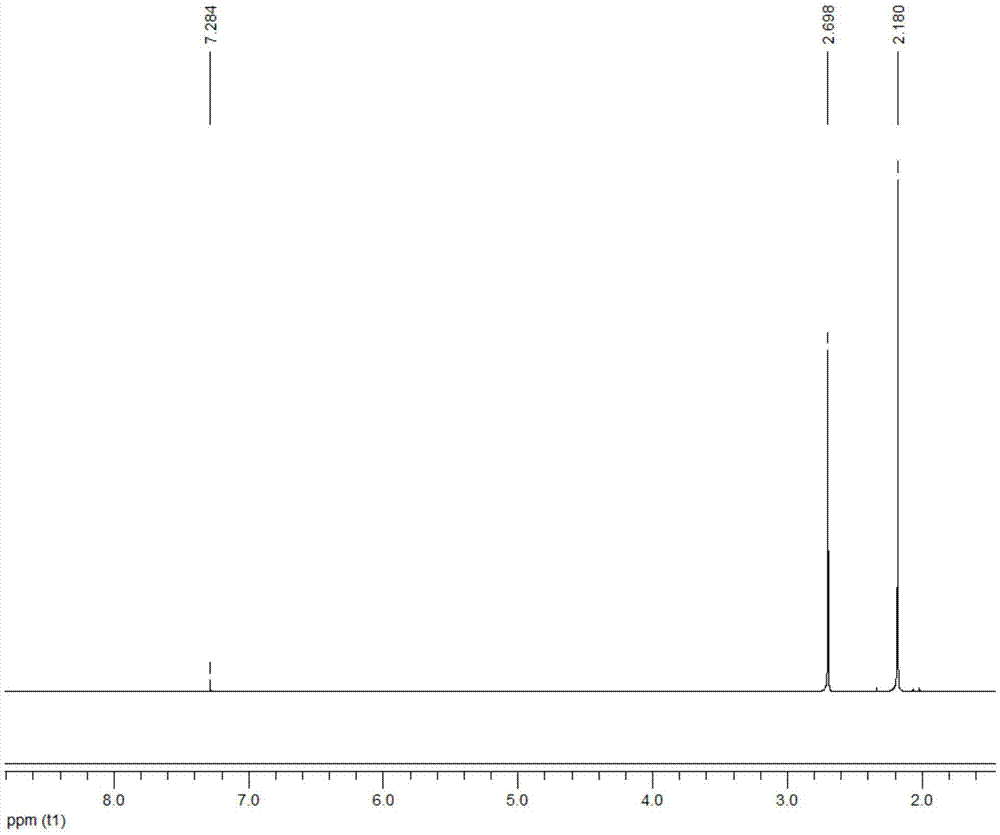
[0028] Add 1ml of 2,5-dimethylfuran into the container, then add 12ml of methyl isobutyl ketone, 0.2ml of deionized water and 0.8g of Hβ molecular sieve (silicon to aluminum ratio of 20), and mix well. The container was placed in a constant temperature reactor at 180° C., stirred continuously, and reacted for 5 hours. After the reaction, the container was put into an ice bath to quench the reaction, and the temperature of the system dropped rapidly to room temperature. The reaction product is taken out, the organic phase and the solid catalyst are separated by centrifugation, the organic phase is collected, and the catalyst is recovered. The organic phase obtained is distilled under reduced pressure, removes organic solvent, obtains product (see attached figure 2 with 3 ). The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography....
Embodiment 2
[0030] First add 1ml of raw material 2,5-dimethylfuran into the container, then add 10ml of methyl isobutyl ketone, 0.8g of USY molecular sieve (silicon-aluminum ratio of 5), and 0.1ml of deionized water. After mixing evenly, put it into a reactor, stir, and react at a constant temperature of 180° C. for 5 hours. After the reaction is completed, the reaction product is quenched in a low temperature, and the temperature is rapidly dropped to normal temperature. Then centrifuge, recover the catalyst, collect the organic phase, distill the obtained organic phase under reduced pressure, remove the organic solvent, and obtain the product. The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography. The results showed that the conversion rate of the raw material 2,5-dimethylfuran was 85%, the purity of the product was 90%, and the yield of 2...
Embodiment 3
[0032] Into the container, sequentially add: 1ml 2,5-dimethylfuran, 12ml methyl isobutyl ketone, 0.1ml deionized water, 1.2g ZSM-5 zeolite molecular sieve (silicon-alumina ratio is 20), and mix evenly. The container was placed in a constant temperature reactor and reacted at 200° C. for 5 hours. After the reaction, the system was rapidly dropped to normal temperature. The reaction solution is separated from the solid phase and the organic phase, the catalyst is recovered, and the organic phase is collected. The organic phase was distilled under reduced pressure to remove the organic solvent to obtain the product. The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography. The results showed that the conversion rate of the raw material 2,5-dimethylfuran was 82%, the purity of the product was 92%, and the yield of 2,5-hexanedione was 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com