A kind of preparation method of 2-carbonyl furan compounds
A carbonylfuran and compound technology, which is applied in the field of preparation of 2-carbonylfuran compounds, can solve the problems of not being able to realize environmental protection and high yield at the same time, and achieve considerable economic effects, important social significance, and easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
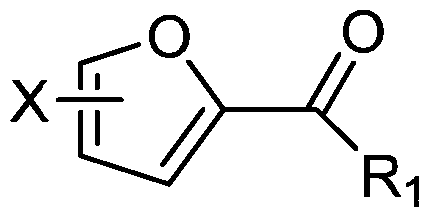
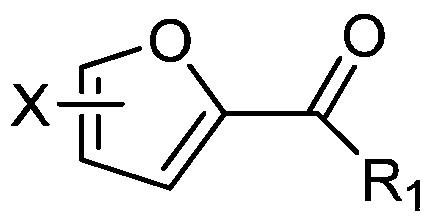
[0016] The present invention provides a kind of preparation method of the 2-carbonylfuran compound of structure shown in formula (I), and this method comprises the following steps:
[0017] (1) In the presence of an acidic catalyst, the furan compound of the structure shown in the formula (II) and the general formula R 1 The aldehyde compound of CHO undergoes condensation reaction;
[0018] (2) Under the condition of oxidation reaction, carry out oxidation reaction with the condensation product of step (1) and oxidizing agent;
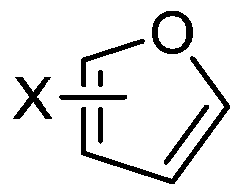
[0019] Formula (I), Formula (II),
[0020] R 1 In CHO and general formula (I), R 1 It is an alkyl group with 1-5 carbon atoms, carboxyl group, an alkyl carboxyl group with 2-6 carbon atoms, or an aldehyde group with 1-5 carbon atoms;
[0021] In formula (I) and formula (II), X is H, an alkyl group with 1-5 carbon atoms, an alkoxy group with 1-5 carbon atoms, acetyl, propionyl, butyryl, pentanoyl, Halogen, hydroxyalkyl group with 1-5 carbon ato...
Embodiment approach
[0039] According to one embodiment, when a solid acid catalyst is used, it is necessary to remove the solids after mixing with the neutralizing agent, which may be a conventional option in the art. For example, it can be removed by filtration. The removal method of the solvent in the neutralized mixture may be conventionally selected in the art, for example, it may be by distillation under reduced pressure.
[0040] According to the present invention, the oxidant used in step (2) can be a conventional selection in the art, for example, the oxidant can be selected from air, oxygen, hydrogen peroxide, TBHP (tert-butyl hydroperoxide), TEMPO (2,2 ,6,6-tetramethylpiperidine-nitrogen-oxide), TEMPO / TiO 2 , PCC (pyridinium chlorochromate), PDC (pyridinium dichromate), SeO 2 , CrO 3 and at least one of potassium permanganate. Preferably, the oxidizing agent is selected from TEMPO, TEMPO / TiO 2 , at least one of oxygen and TBHP.
[0041] In the present invention, the furan compound...
Embodiment 1
[0051] This example is used to illustrate the preparation method of the 2-carbonylfuran compound provided by the present invention.
[0052] Weigh 6.8g of furan and 0.55g of sulfuric acid / diatomaceous earth in a 250ml reaction flask, add 20g of acetonitrile, preheat to 15°C and keep warm for 0.5h, and slowly add 7.4g of glyoxylic acid aqueous solution with a concentration of 40% by weight dropwise to The reaction bottle was stirred vigorously, and after 10 minutes of dropwise addition, the reaction was carried out at 0°C for 5 hours. Based on glyoxylic acid, the conversion rate of furan was 75.6%, and the selectivity of 2-hydroxy-2-furan acetic acid was 84.6%.
[0053] The resulting reaction solution was neutralized and filtered to remove solids, and the low boilers were removed by distillation under reduced pressure, and 20 g of water and 0.5 g of TEMPO / TiO were added. 2 At 40°C, 80ml of hydrogen peroxide with a concentration of 27% by weight was slowly added dropwise to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com