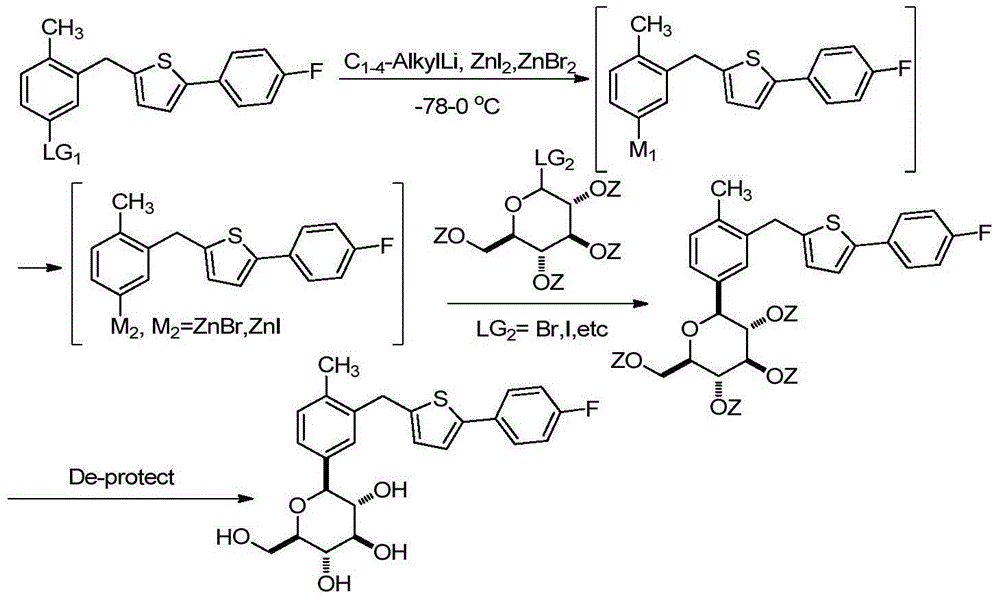
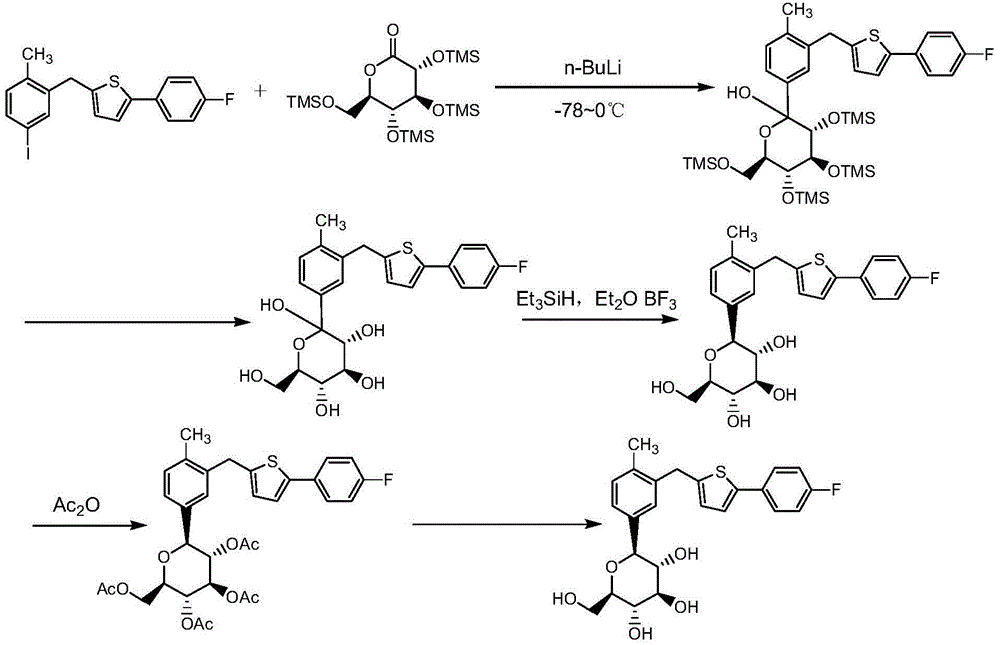
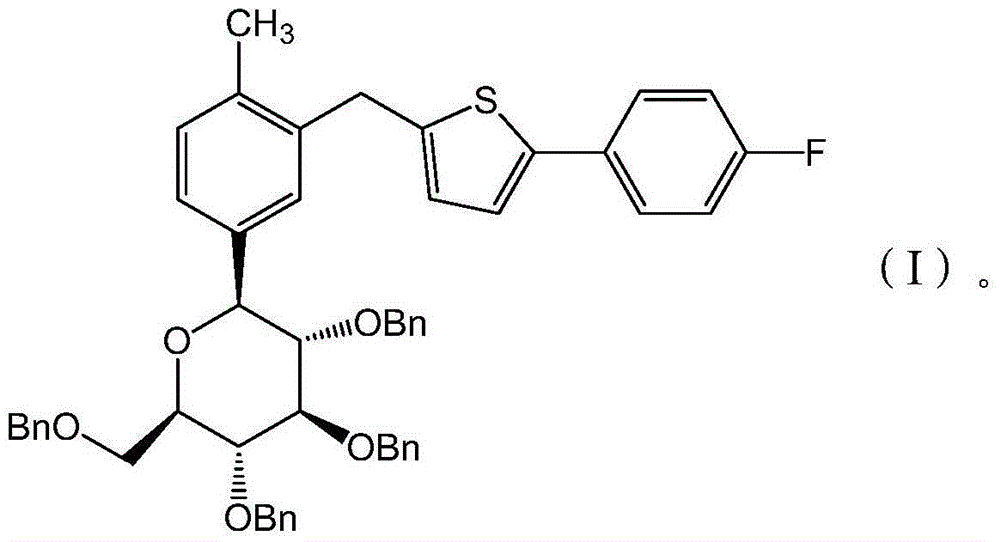
Preparation methods for canagliflozin and intermediate thereof and intermediate
A technology for canagliflozin and intermediates, which is applied in the field of preparing canagliflozin and its intermediates, can solve problems such as complex reaction routes, and achieve the effects of simple reaction routes, product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a preparation method of canagliflozin, comprising the following steps:
[0034] The structural compound shown in formula (I) carries out debenzylation reaction, obtains canagliflozin;
[0035]
[0036] In the present invention, the compound represented by the formula (I) undergoes a debenzylation reaction to obtain canagliflozin. The structural compound shown in the formula (I) is preferably prepared according to the following steps:
[0037] The structural compound shown in the formula (II) is reduced in the presence of a reducing agent to obtain the structural compound shown in the formula (I);
[0038]
[0039] In formula (II), R is selected from hydrogen or methyl.
[0040] The structural compound represented by the formula (II) has a structure of formula (II-I) or formula (II-II);
[0041]
[0042] In the present invention, the compound of formula (II) undergoes a reduction reaction in the presence of a reducing agent to obtain th...
Embodiment 1
[0073] Under the protection of argon, add 300mL of tetrahydrofuran and 90.4g (250mmol) of 2-(2-methyl-5-bromobenzyl)-5-(4-fluorobenzene)thiophene into a 1000mL three-necked flask , use an acetone / dry ice bath to control the temperature of the mixed system at -78°C, add 100mL (2.5mol / L, 250mmol, 1eq) of n-butyl lithium dropwise to the three-necked flask, and control the temperature of the reaction system during the dropwise addition At -78°C, after the dropwise addition was completed, it was left to stand at -78°C for 1 hour. To the reaction system, dropwise add a THF solution of 2,3,4,6-tetra-O-benzyl-D gluconolactone (2,3,4,6-tetra-O-benzyl-D gluconolactone 135.6g, tetrahydrofuran 150mL), stirred while adding dropwise, removed the dry ice / acetone bath after the dropwise addition, slowly returned the temperature of the reaction system to room temperature and reacted for 3 hours. NaHCO 3 The solution quenched the reaction, and the quenched reaction product solution was sequen...
Embodiment 2
[0077] Under the condition of argon protection, 150mL of tetrahydrofuran after dehydration, 300mL of toluene and 114.8g (280mmol) of 2-(2-methyl-5-iodobenzyl)-5-(4-fluorobenzene)thiophene were added to 1000mL In the three-necked flask, use an acetone / dry ice bath to control the temperature of the mixed system at -78°C, add 146mL (2.5mol / L, 364mmol, 1.3eq) of n-butyllithium dropwise to the three-necked flask, and control The temperature of the reaction system does not exceed -40°C. After the addition is complete, let it stand at -78°C for 1 hour, and add 2,3,4,6-tetra-O-benzyl-D gluconic acid to the reaction system dropwise Tetrahydrofuran / toluene solution of lactone (196.2g of 2,3,4,6-tetra-O-benzyl-D gluconolactone, 100mL of tetrahydrofuran, 200mL of toluene), stirring while adding dropwise, withdraw after dropwise addition Dry ice / acetone bath, slowly return the temperature of the reaction system to room temperature and react for 3 hours. After the reaction is stopped, use s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com