Preparation method of insulin detemir or insulin detemir analogue
A technology for insulin detemir and its analogues, which is applied in the field of preparation of insulin analogues, can solve the problems of many by-products, high cost and low acylation efficiency, and achieves the advantages of less by-products, short reaction time and increased overall yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
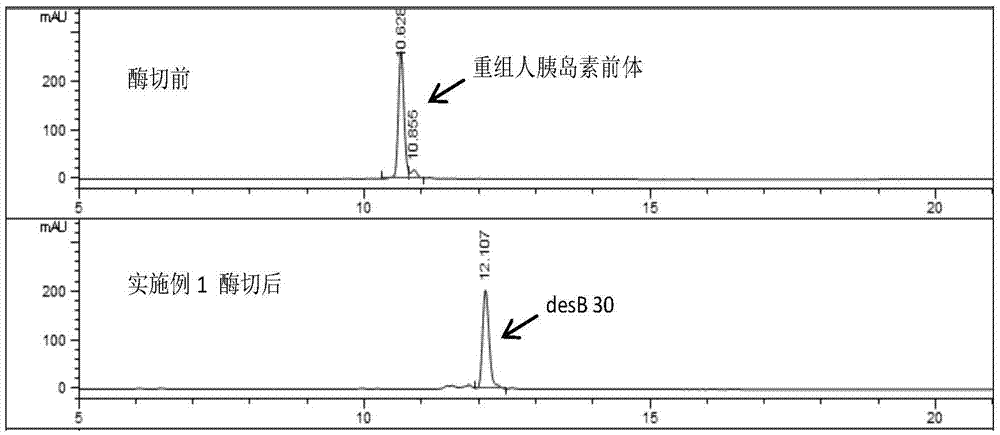
[0032] Embodiment 1 Preparation of recombinant human double-chain insulin precursor (desB30)
[0033] For the preparation of recombinant human double-chain insulin precursor (desB30), refer to relevant literature (Liu Haifeng, Research on the process of converting recombinant insulin precursor into human insulin and insulin detemir, East China University of Science and Technology, Ph.D. dissertation. 2013). details as follows:
[0034] 1.1 Test materials and instruments
[0035] Sodium chloride (NaCl), hydrochloric acid (HCl) and sodium hydroxide (NaOH) were purchased from Sinopharm Chemical Reagent Co., Ltd.; acetonitrile (CH 3 CN, HPLC grade), trifluoroacetic acid (TFA, HPLC grade) were purchased from J&K Chemical Company; the analytical HPLC instrument was Agilent 1260, and the analytical chromatographic column was Kromasil-C18-5μm- (4.6mm * 250mm); The chromatographic purification system is the AKTAexplorer100 chromatographic workstation of Sweden PharmaciaBiotech compa...
Embodiment 2
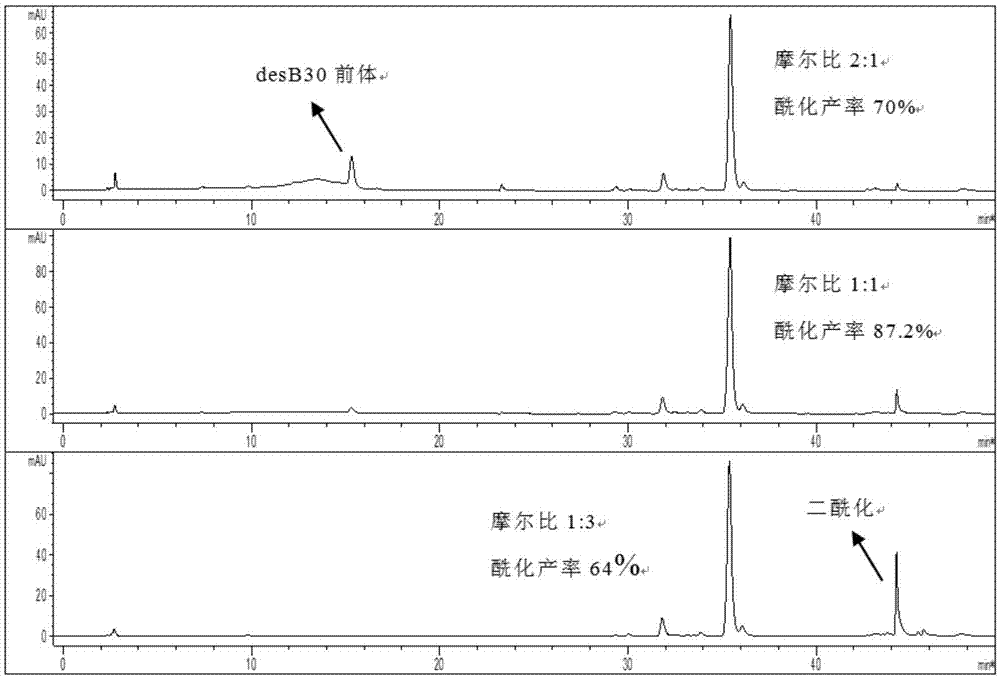
[0047] The preparation of embodiment 2 insulin detemir
[0048] Dissolve the lyophilized powder of recombinant human double-chain insulin precursor desB30 in disodium hydrogen phosphate at 120mmol / LpH2 and boric acid solution at 120mmol / LpH2 respectively, and stir the solution until the lyophilized powder is completely dissolved (observed with the naked eye), so that the final concentration of desB30 Both are 6mg / ml. Dissolve the solid activated ester (succinimide myristate) in pure acetonitrile, and sonicate until it is completely dissolved, so that the concentration of the activated ester is 10 mg / ml.
[0049] Use 1mol / L NaOH to uniformly adjust the pH of the desB30 precursor solution to 9.5, add pure acetonitrile so that the volume occupied by acetonitrile is 50% of the total reaction solution volume, stir slowly with a magnetic stirrer until completely mixed, and slowly add activated ester to make The molar ratio of activated ester to desB30 is about 1:2, stirred for 60 m...
Embodiment 3
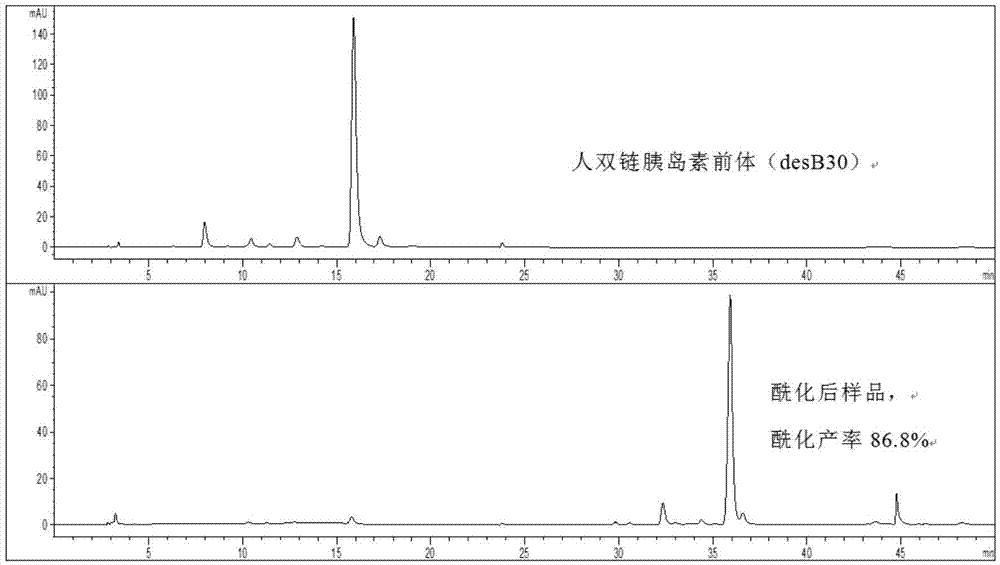
[0052] The preparation of embodiment 3 insulin detemir analogs
[0053] The recombinant human double-chain insulin precursor desB30 lyophilized powder was dissolved in 100mmol / LpH3 disodium hydrogen phosphate solution, and the solution was stirred until the lyophilized powder was completely dissolved (observed with the naked eye), so that the final concentration of desB30 was about 6mg / ml. Dissolve the solid activated ester (N-succinimidyl palmitate) in ethanol, and sonicate until completely dissolved, so that the concentration of the activated ester is 10 mg / ml.
[0054] Use 1mol / L NaOH to adjust the pH of the desB30 precursor solution to 9.5, add pure ethanol so that the volume of ethanol is 40% of the total reaction solution volume, stir slowly with a magnetic stirrer until completely mixed, and slowly add activated ester to make the activated ester The molar ratio to desB30 is about 1:2, and the reaction is stirred for 60 minutes, and the reaction temperature is 25°C.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com