Bacteriophage preparation and application thereof
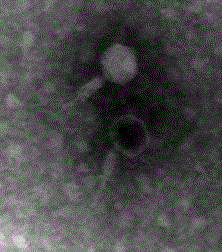
A bacteriophage and preparation technology, applied in the field of bacteriophage preparations, can solve the problems of not providing the lysis ability of piglet diarrhea pathogenic bacteria isolates, not publishing phage transmission electron microscopy images, and unable to effectively control piglet diarrhea, etc. Scope, good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Isolation, amplification and purification of enterotoxigenic Escherichia coli K88 phage
[0024] Phage isolation: CVCC1502 (serum type O9:K88ac) purchased from China Veterinary Microbiology Culture Collection Center was used as host to isolate phage. The bought bacteria were revived and cultivated separately, and a single K88 colony on the LB plate was picked, inoculated in 5 mL of LB medium, shaken at 150 rpm overnight at 37°C, and an overnight bacterial solution was prepared. Fecal and urine sewage samples from pig farms (a pig farm in Jiangsu) were collected, filtered through double-layer filter paper, centrifuged at 12000×g for 20 min, and then the supernatant was filtered with 0.45 μm and 0.22 μm filter membranes respectively; 10 mL of the filtrate was added to 0.5mL overnight cultured host bacteria (the bacterial content is 10 9 cfu / mL), then add 20mL of 4×LB medium, place it at 37°C for 12-16h, and the next day, take the above culture and centrifuge it...
Embodiment 2
[0029] The preparation of embodiment 2 phage preparation JS-15
[0030] Take the Escherichia coli K88 phage EK88-4 obtained in Example 1 and the Escherichia coli K99 phage EK99-C disclosed in the patent ZL200910263295.0, the titers of the two phages are all ≥ 10 8 Pfu / mL, the two phages are fully mixed according to the titer ratio, and an appropriate amount of SM buffer is added to prepare the compound phages of enterotoxigenic Escherichia coli K88 and K99. The preparation is self-named JS-15, and the total content of bacteriophage should be ≥10 6 pfu / g or 10 6 pfu / mL.
[0031] In the actual operation process, one, two or more mixtures of sodium alginate, sucrose, maltodextrin, and glucose can also be added to the phage preparation JS-15, as long as the total content of phage in the obtained preparation is guaranteed ≥10 6 pfu / g or 10 6 pfu / mL can achieve the purpose of the present invention.
Embodiment 3
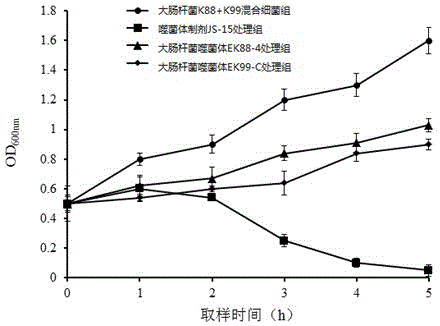
[0032] Phage spectrum analysis of embodiment 3 phage preparation JS-15
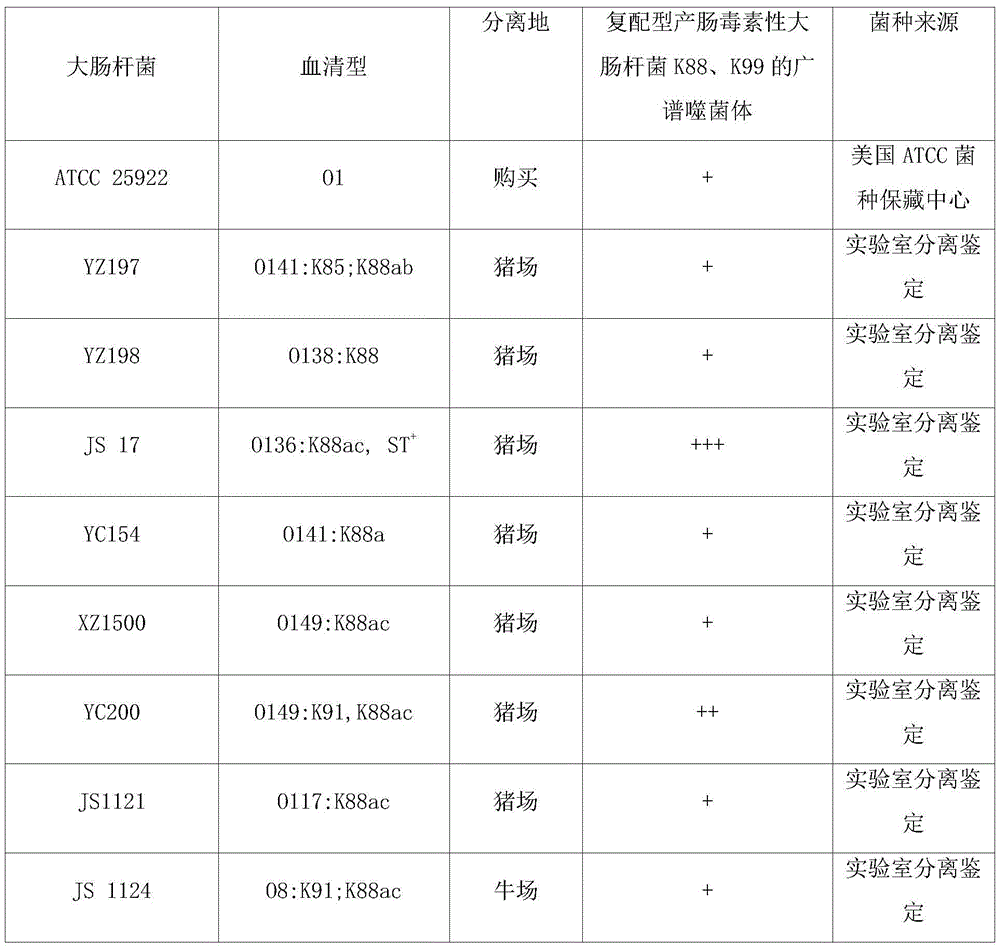
[0033] Take 55 strains of Escherichia coli (see Table 1 for strain sources), recover the strains, cultivate them, and evenly spread 100 μl of the overnight cultured bacterial solution on the LB plate. After drying, take 10 μl of the phage JS-15 prepared in Example 2, Escherichia coli K88 phage EK88-4 and Escherichia coli K99 phage EK99-C were spotted on the surface of the bacterial lawn respectively, and normal saline was used as a control. Each sample was repeated three times, and the droplets were dried and then placed upside down at 37°C for incubation box, cultivated for 12-16 hours and observed the effect the next day. The results are shown in Table 1:
[0034] Table 1 Bactericidal spectrum of phage preparation JS-15 for preventing and treating piglet diarrhea
[0035]
[0036]
[0037]
[0038]
[0039] It can be seen from Table 1 that for the 55 Escherichia coli epidemic strains, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com