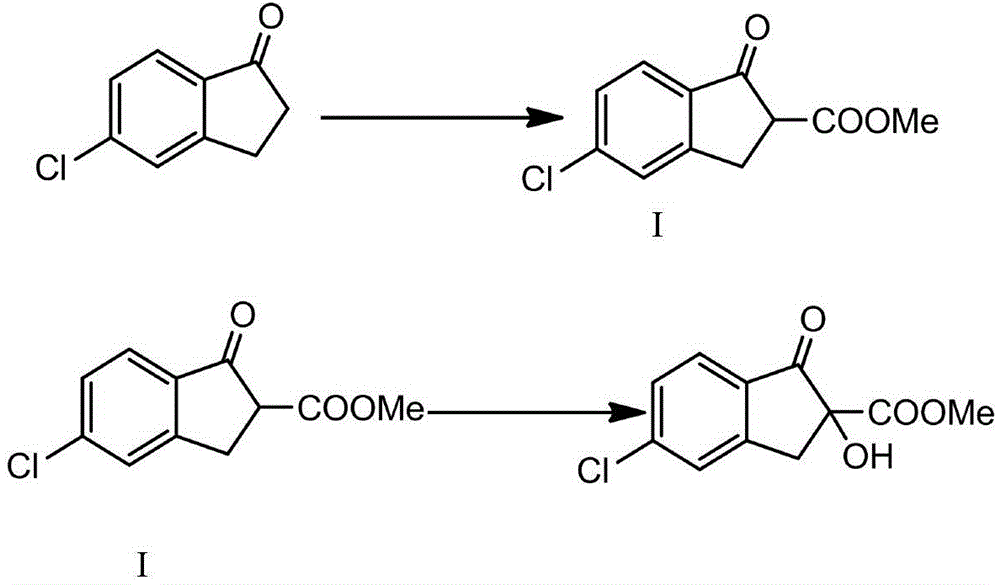
Preparation method of (+)methyl 5-chloro-2,3-dihydro-2-hydroxy-1-oxo-1H-indene-2-carboxylate
A technology of methyl carboxylate and dimethyl carbonate, which is applied in the preparation of carboxylate, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of low optical purity of products, prolonged period, incomplete reaction, etc. The effect of reducing cost, increasing content and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A preparation method of (+)-5-chloro-2,3-dihydro-2-hydroxyl-1-oxo-1H-indene-2-carboxylic acid methyl ester, the specific steps are:
[0019] (1) Add 0.75mol sodium hydride, 1.68mol dimethyl carbonate, 400g toluene to a 1000ml dry flask, heat up to reflux, start to drop 0.375mol 5-chloro-1-indanone, and the dropping time is 1.5h;
[0020] (2) Continue the reflux reaction for 3.5 hours after the dropwise addition is completed. After the reaction is completed, lower the temperature to 25° C., add 36.5 g of concentrated hydrochloric acid and 365 g of ice to the reaction system, stir for 30 min, let stand for liquid separation, and extract the aqueous phase with 200 g of toluene. Washing with water three times, each time with 200ml of water, the washing temperature is 35°C, and then concentrating to remove 50-70% of the mass of toluene to obtain the toluene solution of intermediate I;
[0021] (3) Dissolve 0.03 mol of Sinconin in 48.6 g of toluene, raise the temperature to 4...
Embodiment 2
[0023] (1) Add 0.83mol sodium hydride, 1.32mol dimethyl carbonate, and 313g toluene to a 1000ml dry flask, heat up to reflux, start to drop 0.375mol 5-chloro-1-indanone, and the dropping time is 2.1h;
[0024] (2) Continue reflux reaction for 4 hours after the dropwise addition is completed. After the reaction is completed, lower the temperature to 25°C, add 73g of concentrated hydrochloric acid and 730g of ice to the reaction system, stir for 30min, let stand to separate the liquid, extract the water phase with 200g of toluene, and then wash with water Three times, each time using 200ml of water, washing with water at a temperature of 35°C, and then concentrating to remove 50-70% of the mass of toluene to obtain the toluene solution of intermediate I;
[0025] (3) Dissolve 0.035 mol of Sinkenin in 56.6 g of toluene, raise the temperature to 44°C, add 0.86 mol of cumene hydroperoxide and the toluene solution of intermediate I dropwise to it at the same time, and keep the temper...
Embodiment 3
[0027] A preparation method of (+)-5-chloro-2,3-dihydro-2-hydroxyl-1-oxo-1H-indene-2-carboxylic acid methyl ester, the specific steps are:
[0028] (1) Add 1.0mol sodium hydride, 1.5mol dimethyl carbonate, 386g toluene to a 1000ml dry flask, heat up to reflux, start to drop 0.375mol 5-chloro-1-indanone, and the dropping time is 2h;
[0029] (2) After the dropwise addition, continue to reflux for 3.7 hours. After the reaction is completed, lower the temperature to 25°C, add 54g of concentrated hydrochloric acid and 540g of ice to the reaction system, stir for 30min, let stand for liquid separation, extract the water phase with 200g of toluene, and combine The two toluene phases were then washed three times with 200 ml of water each time at a temperature of 35° C., and then concentrated to remove 50-70% by mass of toluene to obtain a toluene solution of intermediate I;
[0030] (3) Dissolve 0.037 mol of Sinconin in 59.8 g of toluene, raise the temperature to 42°C, add 0.75 mol o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com