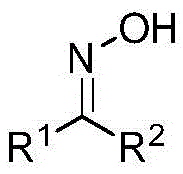
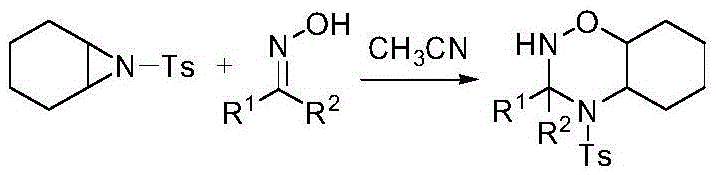
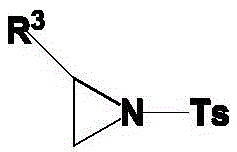
An aziridine compound cyclizing method adopting a ketoxime
An aziridine and compound technology, applied in the field of organic synthesis, can solve the problems of difficult separation of by-products, low yield and the like, and achieve the effects of low reaction cost, high yield and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 0.32mmol of ketoxime 2a, 0.3mmol of aziridine 1a, 20mol% (the mole percentage here is calculated based on the aziridine compound, the same below) KOH with the structural formula shown in Table 1. , acetonitrile 1.5mL, heated to 60°C and stirred for 7h. The crude product was purified by silica gel column chromatography to obtain a single-configuration ring-forming product. The structural formula is shown in Table 1. Characterization confirmed the structure of the product.
[0044] The reaction of table 1 aziridine 1a and acetophenone oxime 2a
[0045]
[0046] 3aWhitesolid; mp120-121℃; 1 HNMR (600MHz, CDCl 3 ):δ7.63-7.64(d,J=8.3Hz,2H),7.54-7.57(m,2H),7.36-7.39(m,3H),7.13-7.15(d,J=8.0Hz,2H), 5.56-5.57(d,J=3.1Hz,1H),3.90-3.95(m,1H),3.11-3.16(m,1H),2.32-2.35(m,4H),2.02-2.06(m,1H), 1.93(s,3H),1.71-1.75(m,1H),1.64-1.66(m,1H),1.31-1.40(m,2H),1.23-1.28(m,2H),ppm; 13 CNMR (150MHz, CDCl 3 ): δ157.8, 145.8, 140.3, 138.8, 132.3, 132.3, 131.4, 130.0, 128.8, 85.6, 60.8, ...
Embodiment 2
[0048] Ketoxime 2b0.45mmol, aziridine 1a0.4mmol, 10mol% K are added to the test tube with the structural formula shown in Table 2 2 CO 3 , acetonitrile 2.0mL, heated to 50°C and stirred for 8h. The crude product was purified by silica gel chromatography to obtain a ring-forming product with a single configuration. The structural formula is shown in Table 2. Characterization confirmed the structure of the product.
[0049] The reaction of table 2 aziridine 1a and oxime 2b
[0050]
[0051] 3b Colourless liquid; 1 HNMR (600MHz, CDCl 3 ): δ7.64-7.66(d, J=8.1Hz, 2H), 7.54-7.56(m, 2H), 7.37-7.38(t, J=3.5Hz, 3H), 7.14-7.16(d, J=8.0 Hz,2H),5.61-5.62(d,J=2.5Hz,1H),3.89-3.94(m,1H),3.12-3.17(m,1H),2.44-2.51(m,1H),2.34-2.37( m,1H),2.33(s,3H),2.03-2.06(m,1H),1.71-1.74(m,1H),1.62-1.66(m,1H),1.32-1.40(m,2H),1.23- 1.31(m,2H),0.96-0.99(t,J=7.4Hz,3H),ppm; 13 CNMR (150MHz, CDCl 3 ): δ162.9, 145.8, 140.2, 137.8, 132.3, 132.2, 131.5, 130.0, 129.0, 85.4, 60.7, 35.7, 33.5, 26.9, 26.7, 24...
Embodiment 3
[0053] Add 0.22mmol of ketoxime 2c, 0.2mmol of aziridine 1a, 25mol% KOH, and 1.2mL of acetonitrile into the test tube, heat to 60°C and stir for 7h. The crude product is purified by silica gel chromatography. A ring-forming product with a single configuration was obtained, the structural formula of which is shown in Table 3 3c, and the structure of the product was confirmed by NMR and high-resolution mass spectrometry.
[0054] Table 3 Reaction of aziridine 1a with oxime 2c
[0055]
[0056]
[0057] 3c Colourless liquid; 1 HNMR (600MHz, CDCl 3 ): δ7.62-7.65(dt, J=2.2,1.9Hz,2H),7.48-7.50(dt,J=2.2,2.3Hz,2H),7.32-7.35(dt,J=2.3,2.3Hz,2H ),7.13-7.15(dd,J=0.6,0.7Hz,2H),5.42-5.43(d,J=3.5Hz,1H),3.89-3.94(m,1H),3.13-3.19(m,1H), 2.34(s,3H),2.28-2.33(m,1H),2.02-2.06(m,1H),1.93(s,3H),1.71-1.75(m,1H),1.63-1.67(m,1H), 1.33-1.41(m,2H),1.27-1.31(m,2H),ppm; 13 CNMR (150MHz, CDCl 3 ): δ156.7, 145.9, 140.4, 138.2, 137.3, 132.3, 131.6, 130.0, 129.9, 85.8, 60.5, 35.9, 33.5, 26.9, 26.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com