Bis(1,3-dithiol-2-carbonyl) fused naphthyl imide derivative and synthesis method thereof
A technology of naphthalenediimide and derivatives, applied in the field of naphthalenediimide derivatives and synthesis thereof, can solve the problems of consumption, occupation, unfavorable aromatic ring system functionalization and derivatization, etc., and achieves simple structure, The effect of simple synthesis route and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
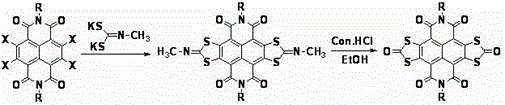
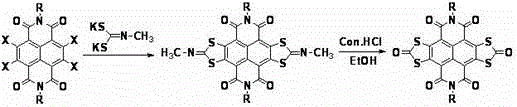
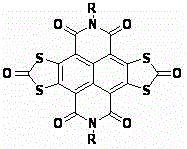
[0033] Add 2,3,6,7-tetrabromonaphthaloyl-n-butylimide (694mg, 1mmol) and N-methylimine potassium dithiocarbonate (1098mg, 6mmol) into a 100mL three-neck round bottom flask ; N 2 Under protection, add 50-60mL of anhydrous THF solvent to the above mixture, reflux and stir for 12h, and cool; transfer the cooled reaction mixture to a single-necked round-bottomed flask, and spin the solvent to dry under reduced pressure; Be 20mL mixed solvent that 1 / 5 joins in the reaction mixture that obtains, and at 90 o C stirred and reacted for 24 hours; the reaction mixture was filtered under reduced pressure, and the solid was washed with deionized water until the filtrate was neutral, and then washed three times with ethanol to obtain a purple-red filter cake; the obtained purple-red solid was dissolved in 30-40 mL of Add 2 to 3 g of silica gel to methyl chloride and mix well, spin the solvent under reduced pressure to obtain a purple solid powder; the purple solid powder is separated and p...
Embodiment 2
[0035] According to the method and steps described in Example 1, only 2,3,6,7-tetrabromonaphthalene diamide (750 mg, 1 mmol) was used instead of 2,3,6,7-tetrabromonaphthalene diamide n-Butylimine (694 mg, 1 mmol), 276 mg of orange-red solid powder was obtained, yield 45%. 1 HNMR (400MHz, CDCl 3 )δ(ppm):0.95-1.05(m,6H,-CH 3 ),1.40-1.56(m,12H,-CH 2 -),1.67-1.82(m,4H,-CH 2 -),4.25-4.35(m,2H,-CH 2 -N).
Embodiment 3
[0037] According to the method and steps described in Example 1, except that N-methylimine potassium dithiocarbonate (1464mg, 8mmol) was used instead of N-methylimine potassium dithiocarbonate (1098mg, 6mmol) to obtain an orange-red solid Powder 232mg, yield 42%. 1 HNMR (400MHz, CDCl 3 )δ(ppm):0.96-1.07(m,6H,-CH 3 ),1.41-1.52(m,4H,-CH 2 -),1.68-1.83(m,4H,-CH 2 -),4.23-4.36(m,2H,-CH 2 -N).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com