Novel technology for producing recombinant adeno associated viruses in pilot test manner
A virus and virus packaging technology, applied in the direction of viruses/phages, microorganisms, sensory diseases, etc., can solve the problems of low virus production titer, low transfection efficiency, low degree of automation, etc., and achieve the effect of improving culture efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
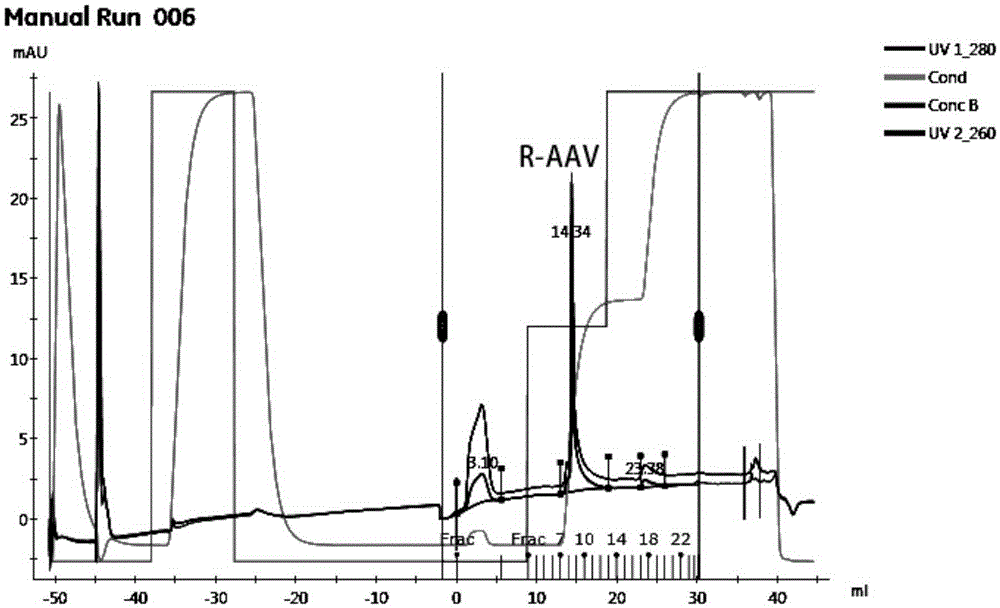
[0028] Example 1. Pilot production of recombinant adeno-associated virus by microcarrier culture of 293 series virus packaging cells using WAVE wave bioreactor
[0029] 1.293TN cell culture
[0030] 1.1 Microcarrier pretreatment: refer to the "Microcarrier Cell Culture: Principles and Methods" (18-1140-62) manual provided by GE Healthcare to perform microcarrier pretreatment.
[0031] 1.2 Cell recovery culture: preheat DMEM medium, take out the cell cryopreservation tube from the liquid nitrogen tank, quickly put it into a water bath filled with 37°C water, and shake it from time to time, thaw as soon as possible and move to the ultra-clean workbench; aseptic operation Aspirate the cell suspension and add it to 3ml of culture medium, then add 5ml of culture medium to dilute, blow it off gently; collect cells by centrifugation at 1000rpm for 3min; resuspend cells in appropriate amount of culture medium for inoculation.
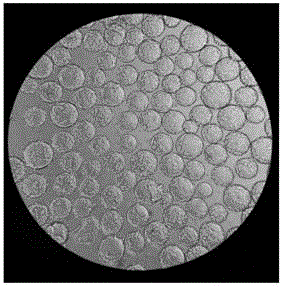
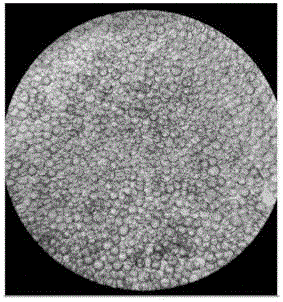
[0032]1.3 Microcarrier culture: use EDTA-trypsin-treated...
experiment example 1
[0040] Experimental Example 1. Suitable Microcarrier Screening Experiment
[0041] Under the premise of fixed inoculation with the same number of 293TN cells, three groups of experiments were set up to investigate the effects of microcarriers Cytodex1, Cytodex2 and Cytodex3 on cell proliferation. Three parallel experiments were set up for each group of investigation experiments, and the initial cell seeding density of each experiment was 3×10 5 Each / mL, and the amount of added microcarriers Cytodex1, Cytodex2 and Cytodex3 were all 3g / L.
[0042] The experimental results are shown in Table 1. It can be seen from the table that after 24h and 48h of microcarrier culture, the cell density has increased. However, the 293TN cells cultured with different microcarriers had some differences in their cell density changes, especially when the 293TN cells were cultured with the microcarrier Cytodex2, the density of the cells did not increase significantly; , 48h later, the cell density...
experiment example 2
[0045] Experimental example 2. Microcarrier dosage optimization experiment
[0046] Taking microcarrier Cytodex1 and microcarrier Cytodex3 as optimization objects, the dosage is set to 1g / L, 2g / L, 3g / L, 4g / L, 5g / L, 6g / L, 7g / L, and the initial cell seeding density is 3 ×10 5 cells / mL, cultured to 48h and sampled for cell counting.
[0047] The experimental results are shown in Table 2. It can be seen from the table that with the increase of the amount of microcarriers, the cell density after 48 hours of culture gradually increased. 6 pcs / ml~2.7×10 6 cells / ml, if the amount of microcarriers continues to increase, the cell density will show a downward trend. Therefore, the present invention determines that the fixed initial seeding cell density is 3×10 5 In the case of cells / mL, the optimal dosage of microcarriers Cytodex1 and Cytodex3 for 293TN cell culture is 2g / L-5g / L.
[0048] Table 2. Effect of microcarrier dosage on cell growth
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com