A method of separating and purifying quercetagetin from tagetes erecta
A technology for separation and purification of quercetin marigold, which is applied in the direction of organic chemistry, can solve the problems of unsuitable separation and purification of substances with complex components, unsuitable for industrialized large-scale production, and lack of repeatability, so as to shorten the preparation time and batch High degree of consistency between times and the effect of reducing column elution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The method for separating and purifying quercetin tagetin from marigold in this embodiment comprises the following steps:
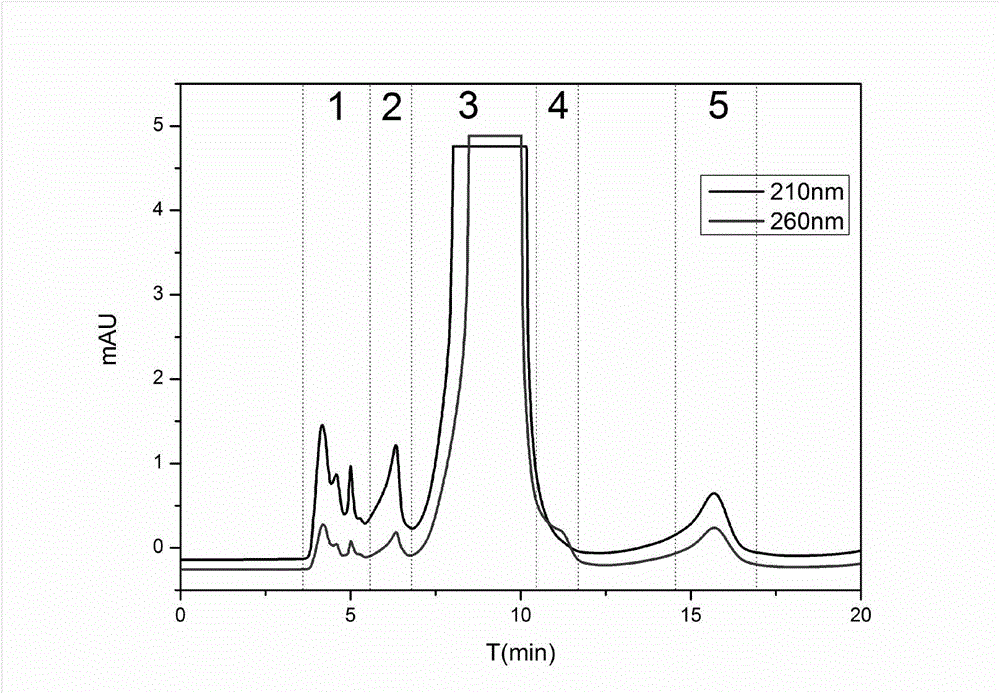
[0037] Take dried marigold flowers as raw materials, grind them into powder, extract and rotary steam with ethanol to obtain dried marigold coarse powder, weigh 5g, dissolve in 50mL of 95% methanol-water solution, and prepare a marigold extract solution with a concentration of 100mg / mL, passed through a 0.45 μm microporous membrane, and prepared by one-dimensional liquid chromatography. One-dimensional liquid chromatography using reversed-phase column DAISOC 18 (250mm×20mmi.d., 8μm), the mobile phase is a mixture of organic phase and water phase, wherein phase A is water, phase B is methanol, isocratic elution method: concentration of phase B is 53% isocratic for 20min. Using UV detector 210nm and 260nm as dual selective absorption wavelengths, preparation temperature is room temperature, injection volume is 500μL / needle, mobile phase flow rate i...
Embodiment 2
[0040] The method for separating and purifying quercetin tagetin from marigold in this embodiment comprises the following steps:
[0041] Take dried marigold flowers as raw materials, grind them into powder, extract and rotary steam with ethanol to obtain dry marigold coarse powder, weigh 5g, dissolve in 100mL95% methanol-water solution, and prepare marigold extract solution with a concentration of 25mg / mL, passed through a 0.45 μm microporous membrane, and prepared by one-dimensional liquid chromatography. One-dimensional liquid chromatography using reversed-phase column DAISOC 18 (250mm×20mmi.d., 8μm), the mobile phase is a mixture of organic phase and water phase, wherein A phase is water, B phase is methanol, isocratic elution method: B phase concentration 55% isocratic 20min, 0 % isocratic for 10 min. Adopting ultraviolet detector 210nm and 260nm as dual selective absorption wavelengths, preparation temperature is room temperature, injection volume is 500 μ L / needle, m...
Embodiment 3
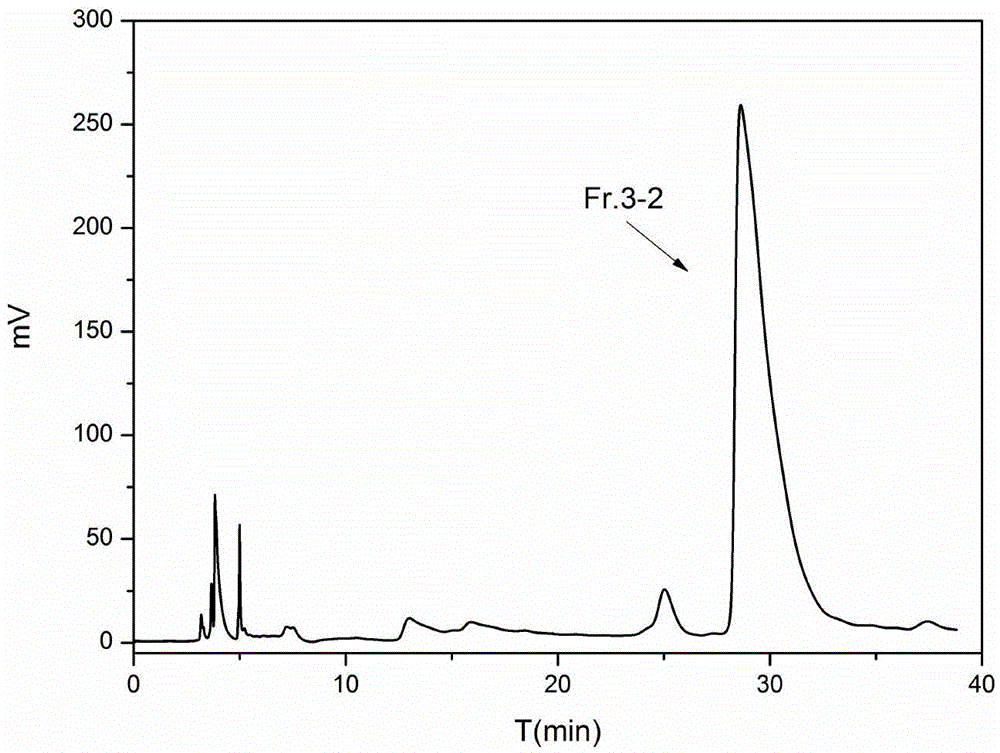
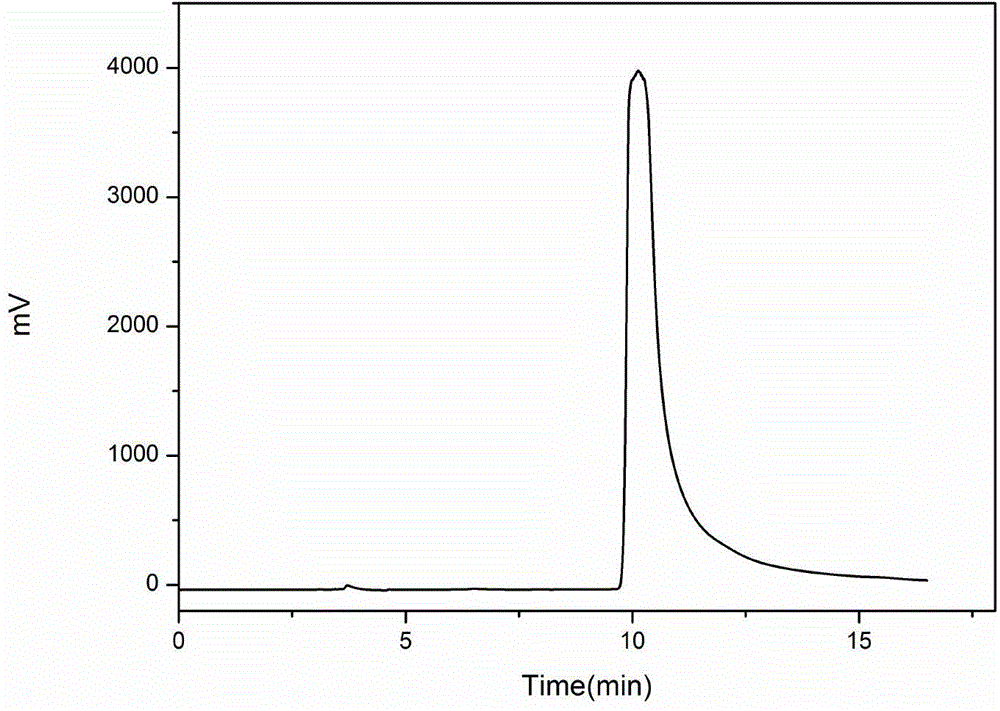
[0044] Take dried marigold flowers as raw materials, grind them into powder, extract and rotary steam with ethanol to obtain dried marigold coarse powder, weigh 5g, dissolve in 25mL of 95% methanol-water solution, and obtain a marigold extract solution with a concentration of 200mg / mL, passed through a 0.45 μm microporous membrane, and prepared by one-dimensional liquid chromatography. One-dimensional liquid chromatography using reversed-phase column DAISOC 18 (250mm×20mmi.d., 8μm), the mobile phase is a mixture of organic phase and water phase, wherein A phase is water, B phase is methanol, isocratic elution method: B phase concentration 50% isocratic 20min, 100 % isocratic for 10 min. Adopting ultraviolet detector 210nm and 260nm as dual selective absorption wavelengths, preparation temperature is room temperature, injection volume is 500 μ L / needle, mobile phase flow rate is 12mL / min, according to ultraviolet absorption spectrum ( Figure 7 As shown), the fractions colle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com