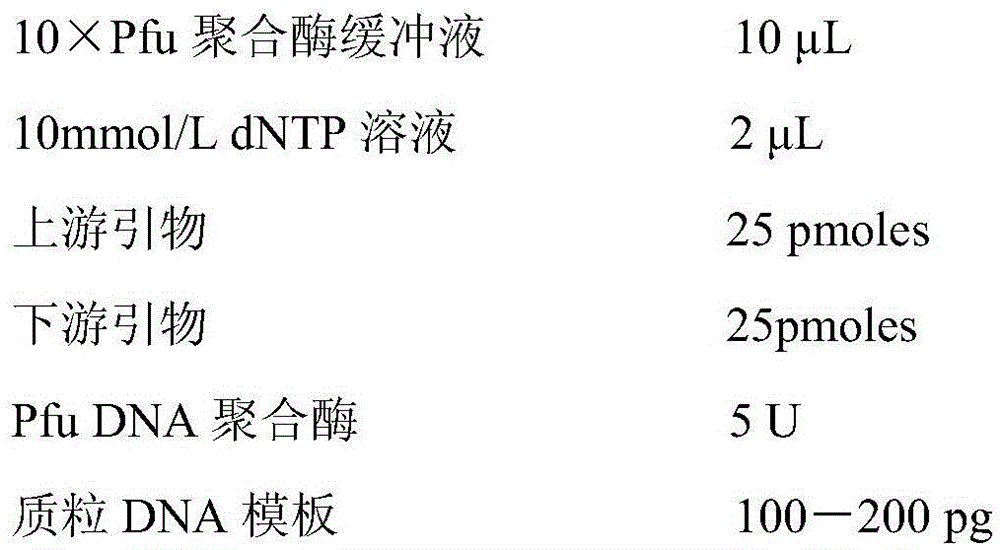
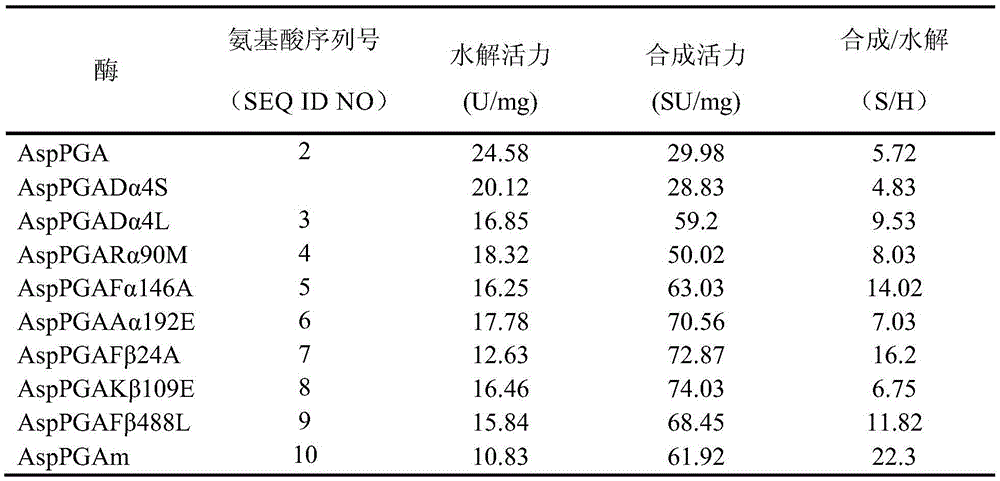
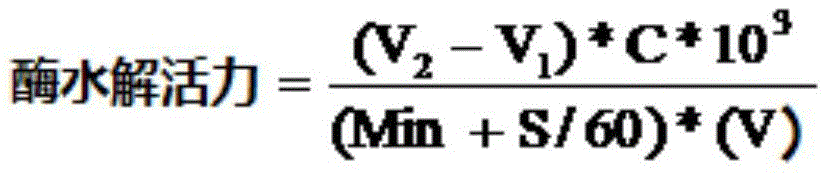Penicillin G acylase mutant
A technology of acylase and penicillin, applied in the field of genetic engineering, can solve the problems of low ratio, reduction of antibiotics, low conversion rate of mother nucleus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Construction of embodiment 1 mutant plasmid
[0092] 1.1. Primer design
[0093] According to the gene sequence SEQIDNO:2 of PGA derived from Achromobacter, and selected 7 mutation sites α4, α90, α146, α192, β24, β109, β488, the following 16 mutation primers were designed:
[0094] Table 1. Construction of mutant primers for different mutant AspPGA enzyme genes
[0095] mutation site
Mutation primer name
Primer sequence (5'-3')
Dα4
Dα4S F1
ACGGCCCCAAACCGCCTCGGGCAAGGTCACGAT
Dα4
Dα4S F2
ATCGTGACCTTGCCCGAGGCGGTTTGGGGCCGT
Dα4
Dα4L F1
ACGGCCCCAAACCGCCCTGGGCAAGGTCACGAT
Dα4
Dα4L F2
ATCGTGACCTTGCCCAGGGCGGTTTGGGGCCGT
Rα90
Rα90M F1
TGCCGGCCGCCGACATGCAGGTGCTGGA
Rα90
Rα90M F2
TCCAGCACCTGCATGTCGGCGGCCGGCA
Fα146
Fα146A F1
ACCATGGCCAACCGCGCTTCGGACGCCAACAGCGA
Fα146
Fα146A F2
TCGCTGTTGGCGTCCGAAGCGCGGTTGGCCATGGT
Aα192
Aα192E F1
CGCCGACCAC...
Embodiment 2
[0115] The acquisition of embodiment 2 engineering bacteria
[0116] With reference to "Molecular Cloning Experiment Guide" (third edition), edited by J. Sambrook, D.W. Russell (US), translated by Huang Peitang, etc., Science Press, Beijing, 2002, page 96 of the first chapter, respectively Nine kinds of plasmids (AspPGADα4S, AspPGADα4L, AspPGARα90M, AspPGAFα146A, AspPGAAα192E, AspPGAFβ24A, AspPGAKβ109E, AspPGAFβ488L and AspPGAm) were transformed into E. Spread on the LB selective plate added with kanamycin sulfate antibiotic, and incubate upside down at 37°C for about 12-18 hours. Those that can grow on the LB plate added with kanamycin sulfate antibiotic are transformants transformed with recombinant plasmids.
Embodiment 3
[0117] Embodiment 3 Engineering bacteria fermentation culture
[0118] Pick a single colony from the LB selective culture plate, inoculate it into 3 mL of LB liquid medium, add kanamycin to a final concentration of 100 μg / mL, and culture at 250 r / min at 37°C overnight; take 2 mL and culture overnight Inoculated into 200mL of LB liquid medium, cultured at 250r / min at 37°C for 4-6h, OD 600 When it reaches 1.0-1.6, the seed bacterial liquid is obtained. Insert the inoculation amount of 1:15 into a 5-liter fermenter containing 3 liters of TB medium, and ferment to OD at 37°C, 400rpm, and 1.3vvm fermentation conditions 600 When it reaches 1.0 (about 1 hour), adjust the temperature to 30°C, induce with a final concentration of 1% lactose, continue to cultivate for 2 hours, then add a final concentration of 0.5% lactose to induce, (the final concentration of lactose reaches 1.5%), Continue to cultivate for about 14-16 hours, and the fermentation ends. The fermentation broth was ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com