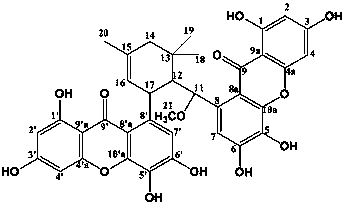
Xanthenone dimer compound IUE-1799a use
A technology of xanthone dimer and iue-1799a, which can be applied to medical preparations containing active ingredients, antibacterial drugs, organic active ingredients, etc., and can solve problems such as penicillin resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation and identification of compound xanthone dimer compound IUE-1799a
[0027] Take 2 kg of the bark part of Bamboo basilica, dry it, soak it in 10L ethyl acetate overnight, repeat it three times, combine the ethyl acetate extracts, filter, and concentrate under reduced pressure to obtain 210 g of the total crude extract extract. The obtained extract was mixed with silica gel (100 mesh) and subjected to normal pressure silica gel (200-300 mesh) column chromatography, using chloroform-methanol as the eluting solvent, and performing gradient elution from 90:10 to 40:60, wherein Gradient elution components that can be developed in thin layer chromatography with a chloroform-methanol-0.1% formic acid aqueous solution system with a volume ratio of 100:20:2 are purified by a medium-pressure reversed-phase column (60 μm), and then passed through SephadexLH -20 column purification, eluting with methanol, 10ml of eluent as a tube, collecting 15 tubes, picking t...
Embodiment 2
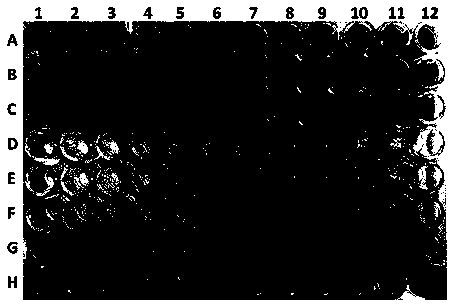
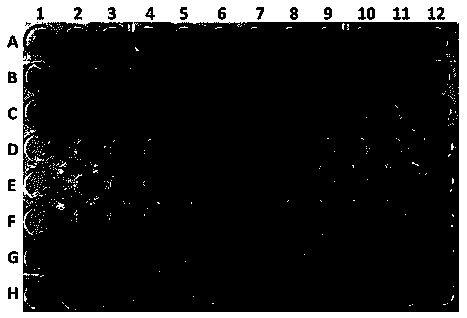
[0029] Embodiment 2: antibacterial activity experiment
[0030] The resazurin chromogenic method was used as the method for detecting the activity. The principle of the resazurin chromogenic method is that lactate dehydrogenase in living cells can convert resazurin (blue) into the fluorescent substance resorufin (pink red) to generate a fluorescent signal. Resorufin will continue to be reduced by the cells to the non-fluorescent substance dihydroresazurin (white), which will decrease the fluorescent signal, and inactive or dead cells will lose their metabolic capacity and cannot restore resazurin, so they will not be able to produce fluorescence Signal, so this method can specifically detect active cells.
[0031] The antibacterial experiment was performed in a 96-well plate, and the color in the 96 wells was blue, indicating that the sample had inhibitory activity against the target strain; the color in the 96 wells was pink or red, indicating that the sample had no inhibito...
Embodiment 3
[0067] Embodiment 3: Cytotoxicity experiment
[0068] In this embodiment, an in vitro cytotoxicity test is used to determine the survival rate of human or mouse cancer cells cultured in vitro after adding IUE-1799a.
[0069] The selected cell lines are: human non-small cell lung cancer H1299, A549, human large cell lung cancer H460, human small cell lung cancer H446, mouse melanoma B16, B16-F10, human breast cancer MD-MBA-231, MCF7, human gastric cancer MGC803 , human pancreatic cancer PANC-1, human cervical cancer HeLa, human osteosarcoma U2OS, human liver cancer HepG2, human colon cancer HCT116, and human laryngeal cancer HEP2. Use the MTT method to determine the half-inhibitory concentration IC when the survival rate (or death rate) of each tumor cell is 50%. 50 (The above cells can be purchased from ATCC). MIC is the minimum inhibitory concentration.
[0070] The test results are shown in Table 2.
[0071]
[0072] It can be seen from Table 2 that IUE-1799a h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com