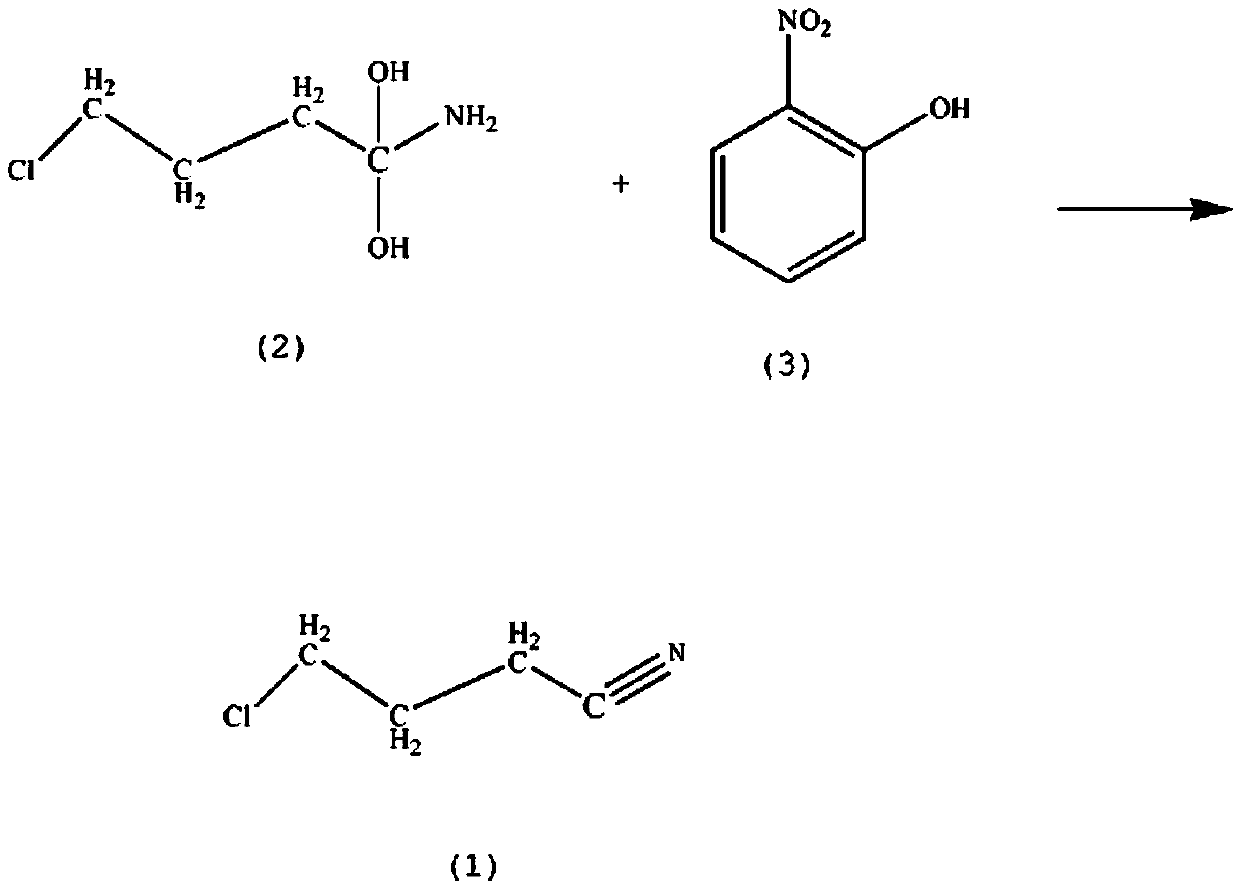
Method for synthesis of haloperidol drug intermediate 4-chlorobutyronitrile
The technology of a kind of haloperidol and synthetic method is applied in the synthetic field of haloperidol drug intermediate 4-chlorobutyronitrile, which can solve the problems of weak sedative effect, reduce intermediate links, reduce reaction temperature and reaction time, The effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] Add 1.6mol of 1-chloro-4,4-dihydroxybutylamine (2) in the reaction vessel, the mass fraction is 60% 2-nitrophenol solution (3) 2.1mol, 1.5mol of aluminum oxide, the mass fraction is 30% Cyclohexane 300ml, control the stirring speed at 130rpm, raise the solution temperature to 90°C, keep reflux for 2h, lower the solution temperature to 70°C, continue the reaction for 90min, lower the solution temperature to 6°C, let stand for 70min, the solution is separated, take out The oil layer is extracted 5 times with 50% toluene in mass fraction, washed with ammonium chloride solution, washed with 70% acrylonitrile in mass fraction, washed with 65% triethylamine in mass fraction, dehydrated with anhydrous potassium carbonate, 90% in mass fraction Recrystallized in nitromethane to obtain 136.45 g of crystalline 4-chlorobutyronitrile with a yield of 82%.
example 2
[0014] Add 1.6mol of 1-chloro-4,4-dihydroxybutylamine (2) in the reaction vessel, the mass fraction is 62% 2-nitrophenol solution (3) 2.3mol, 1.5mol of aluminum oxide, the mass fraction is 32% Cyclohexane 300ml, control the stirring speed at 150rpm, raise the solution temperature to 92°C, keep reflux for 3h, lower the solution temperature to 72°C, continue the reaction for 95min, lower the solution temperature to 7°C, let stand for 80min, the solution is separated, take out The oil layer is extracted 6 times with 52% toluene in mass fraction, washed with ammonium chloride solution, washed with 72% acrylonitrile in mass fraction, washed with 67% triethylamine, dehydrated with solid sodium hydroxide, 93% in mass fraction Recrystallized in nitromethane to obtain 141.44 g of crystalline 4-chlorobutyronitrile with a yield of 85%.
example 3
[0016] Add 1.6mol of 1-chloro-4,4-dihydroxybutylamine (2) in the reaction vessel, the mass fraction is 65% 2-nitrophenol solution (3) 2.6mol, 1.5mol of aluminum oxide, the mass fraction is 35% Cyclohexane 300ml, control the stirring speed at 170rpm, raise the solution temperature to 97°C, keep reflux for 4h, lower the solution temperature to 77°C, continue the reaction for 110min, lower the solution temperature to °C, stand still for 90min, the solution is separated, take out The oil layer is extracted 7 times with 55% toluene in mass fraction, washed with ammonium chloride solution, washed with 75% acrylonitrile in mass fraction, washed with 68% triethylamine, dehydrated with anhydrous potassium carbonate, and 98% in mass fraction Recrystallized in nitromethane to obtain 153.09 g of crystalline 4-chlorobutyronitrile with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com