Dibenzo selenophene derivative and preparation method thereof
A technology of dibenzoselenophene derivatives and benzoselenophene derivatives, which is applied in the field of dibenzoselenophene derivatives and its preparation, can solve the problems of small lethal dose, limitation, and high toxicity of inorganic selenium supplements. Achieve broad application prospects and complex and diverse structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
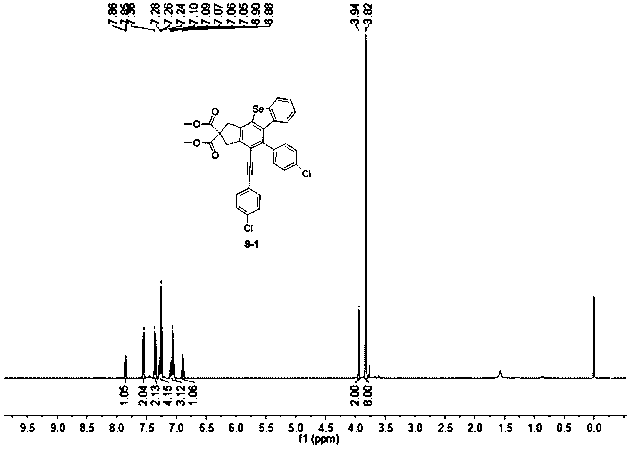
[0044] A dibenzoselenophene derivative, the structural formula of the dibenzoselenophene derivative is:
[0045]
[0046] The preparation method of above-mentioned dibenzoselenophene derivative, comprises the following steps:
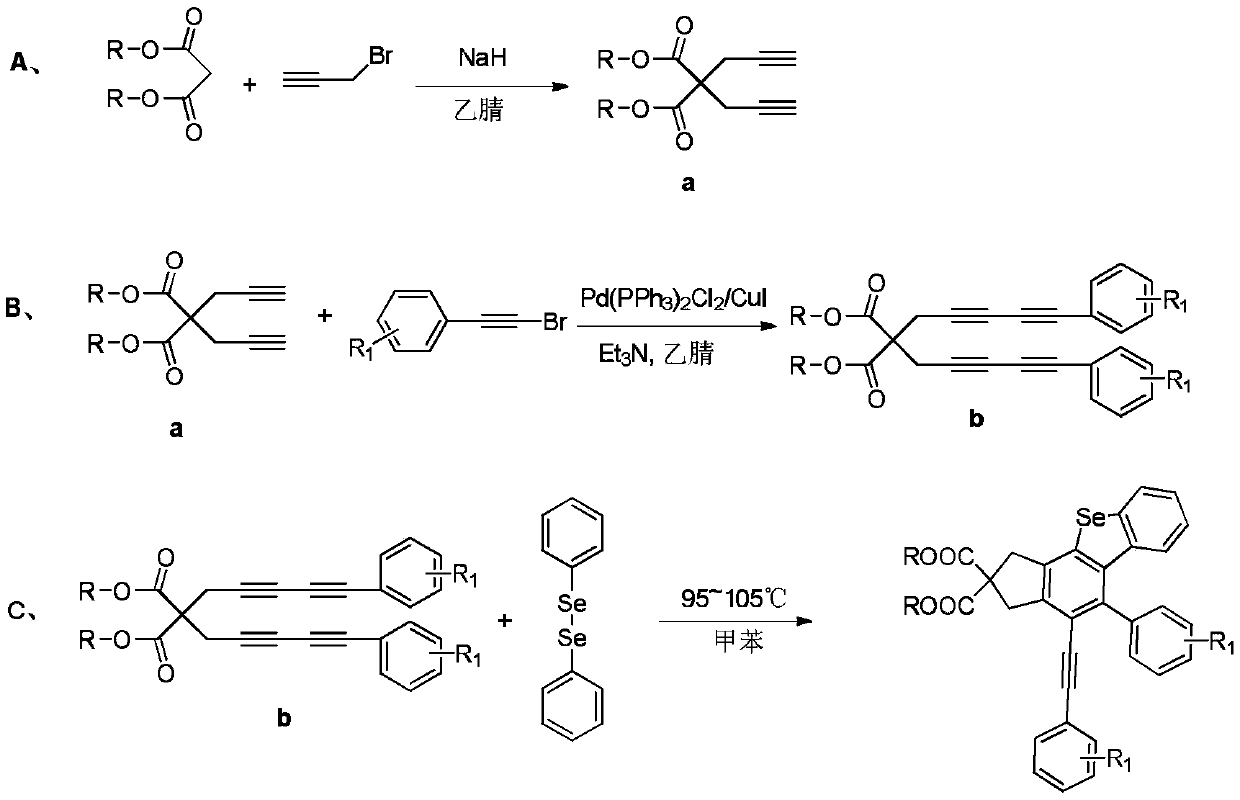
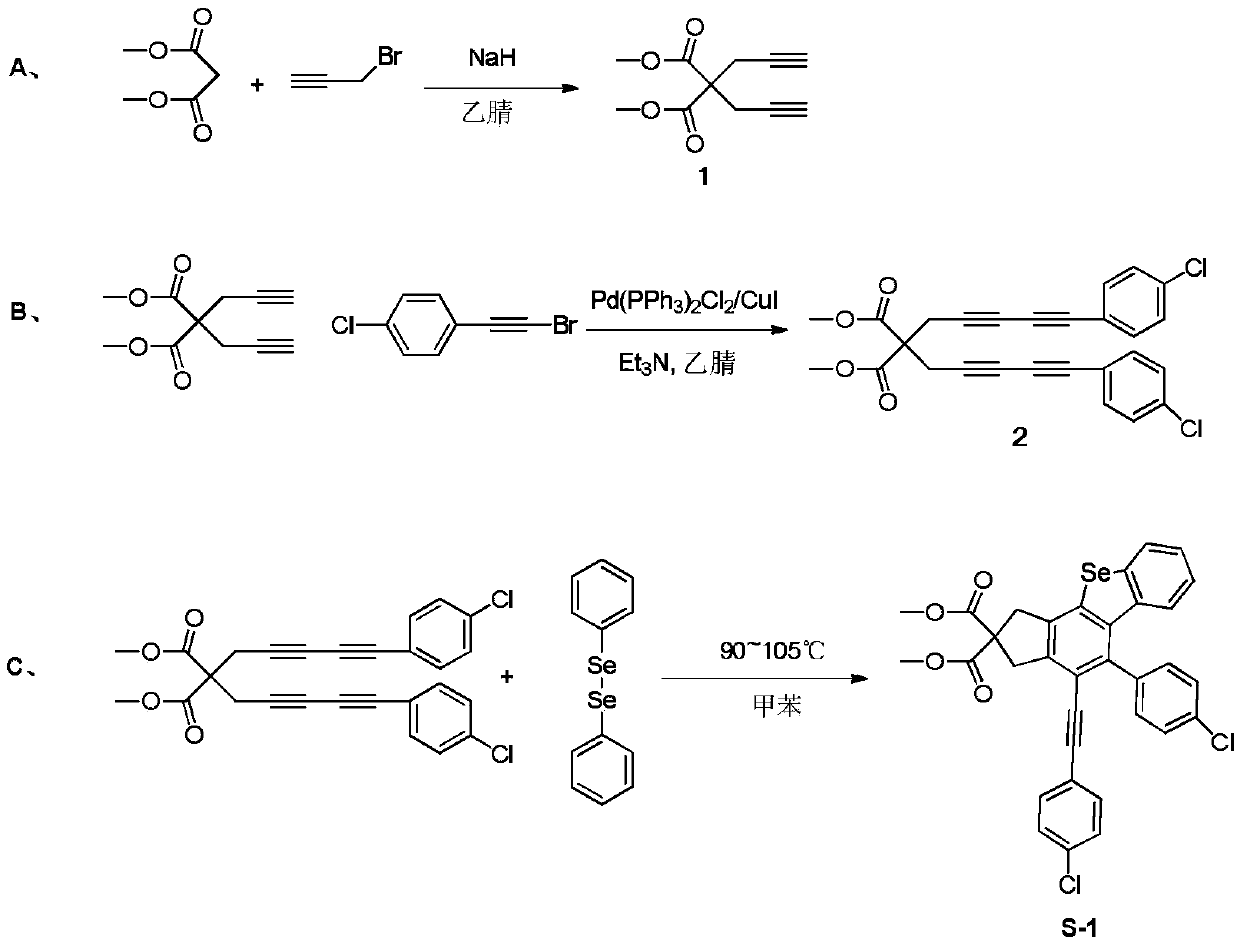
[0047] A. Using sodium hydride (820mmol, 19.7g) as a catalyst, dimethyl malonate (200mmol, 26.6g) and propargyl bromide (450mmol, 53.5g) were added to 250mL of anhydrous acetonitrile, stirred in an ice-water bath After reacting for 8 hours, the crude product was extracted with water and ethyl acetate, concentrated under reduced pressure, separated and purified by silica gel column chromatography, and the eluent was ethyl acetate:petroleum ether=1:100 to obtain a white solid product, namely compound 1;
[0048] B. Compound 1 (80mmol, 16.7g) was mixed with phenylbromoacetylene substitute (176mmol, 37.9g), and in 1.3gPd(PPh 3 ) 2 Cl2 / CuI anhydrous and oxygen-free catalytic system, Pd(PPh 3 ) 2 Cl 2 The molar ratio of CuI and CuI was 3:1, with trie...
Embodiment 2
[0055] A dibenzoselenophene derivative, the structural formula of the dibenzoselenophene derivative is:
[0056]
[0057] The preparation method of above-mentioned dibenzoselenophene derivative, comprises the following steps:
[0058] A. Using sodium hydride (700mmol, 16.8g) as a catalyst, dimethyl malonate (200mmol, 26.6g) and propargyl bromide (440mmol, 52.3g) were added to 330mL of anhydrous acetonitrile, stirred in an ice-water bath After reacting for 10 hours, the crude product was extracted with water and ethyl acetate, concentrated under reduced pressure, separated and purified by silica gel column chromatography, and the eluent was ethyl acetate:petroleum ether=1:60 to obtain a white solid product, namely compound 1;
[0059] B. Compound 1 (80mmol, 16.7g) was mixed with phenylbromoacetylene substitute (176mmol, 37.9g), and in 3.0gPd(PPh 3 ) 2 Cl 2 / CuI anhydrous and oxygen-free catalytic system, Pd(PPh 3 ) 2 Cl 2 The molar ratio of CuI and CuI was 3:1, with tr...
Embodiment 3
[0063] A dibenzoselenophene derivative, the dibenzoselenophene derivative structural formula is:
[0064]
[0065] The preparation method of above-mentioned dibenzoselenophene derivative, comprises the following steps:
[0066] A. Using sodium hydride (810mmol, 19.4g) as a catalyst, (200mmol, 37.6g) diisopropyl malonate and propargyl bromide (500mmol, 59.5g) were added to 250ml of anhydrous acetonitrile, placed in an ice-water bath The reaction was stirred for 10 hours, the crude product was extracted with water and ethyl acetate, concentrated under reduced pressure, separated and purified by silica gel column chromatography, the eluent was ethyl acetate:petroleum ether=1:100, and a white solid product was obtained, namely compound 3 ;
[0067] B. Compound 3 (80mmol, 21.1g) and phenylbromoacetylene substitute (200mmol, 39.0g) were mixed in 2.17gPd (PPh 3 ) 2 Cl 2 / CuI anhydrous and oxygen-free catalytic system, Pd(PPh 3 ) 2 Cl 2 Pd(PPh in / CuI 3 ) 2 Cl 2 The molar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com