A method for oxidizing olefins
A technology for oxidizing olefins and olefins, applied in chemical recovery, organic chemistry, etc., can solve the problems of reduced catalytic performance of catalysts, deactivation of catalysts, inability to obtain the selectivity of target oxidation products, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
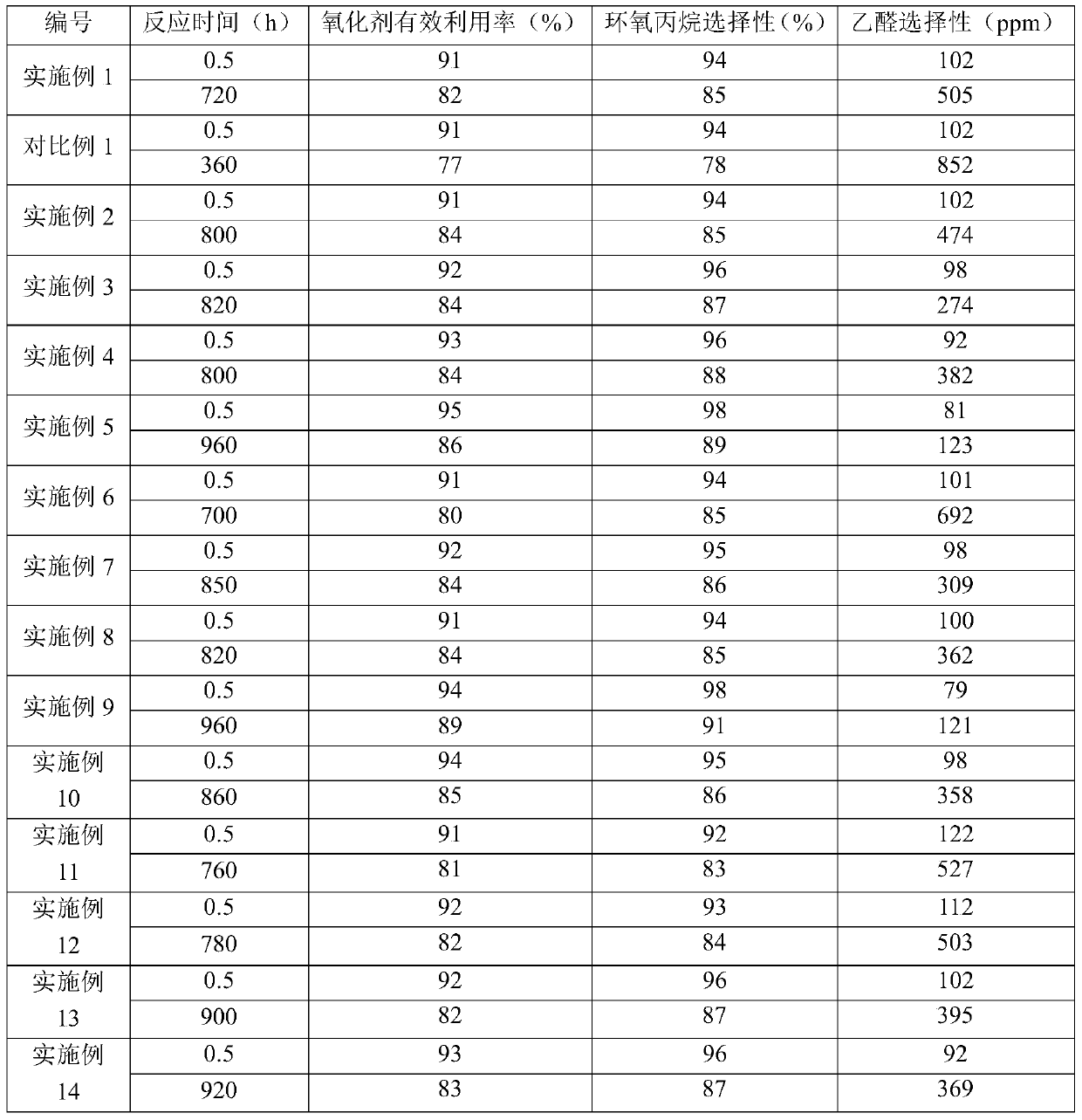
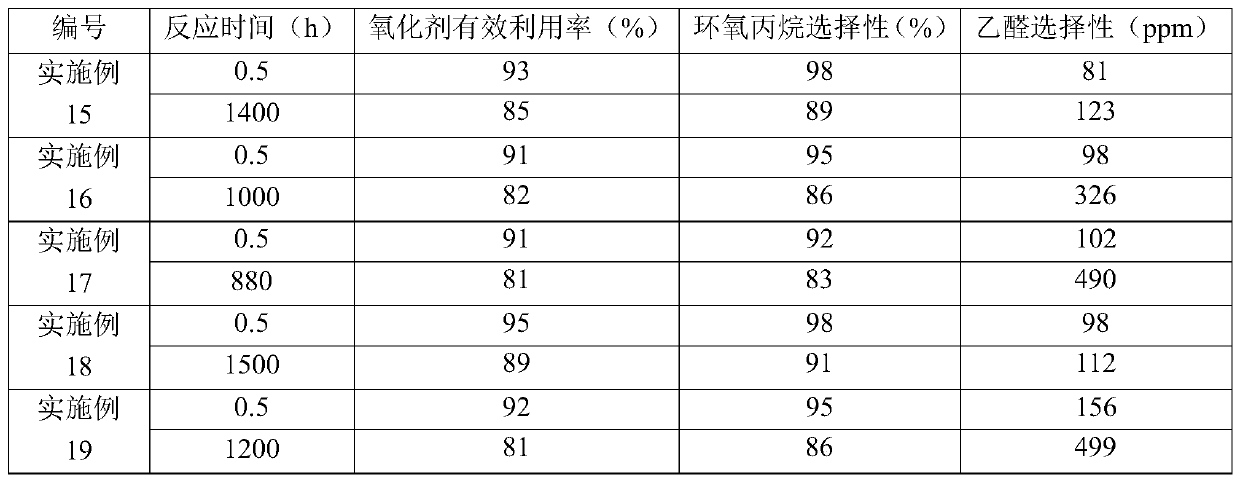
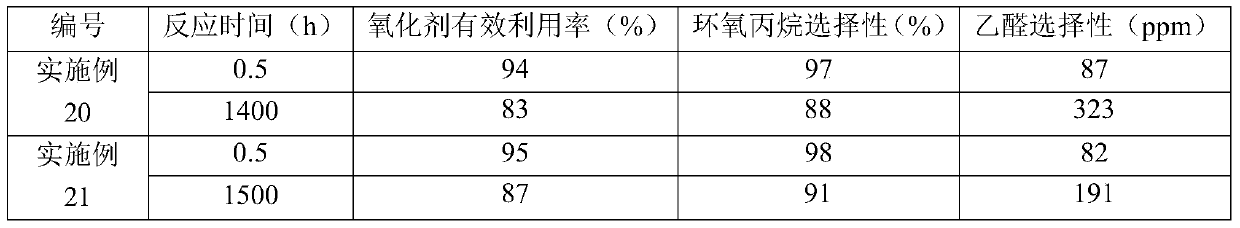
[0099] Catalyst (that is, shaped titanium-silicon molecular sieve TS-1 is a spherical catalyst with a volume average particle diameter of 1000 μm, the content of titanium-silicon molecular sieve TS-1 in the catalyst is 75% by weight, the content of silicon oxide is 25% by weight, and the density is 0.79g / cm 3 ) is packed in a fixed-bed reactor to form a catalyst bed, wherein the number of the catalyst bed is 1 layer.
[0100] Propylene, hydrogen peroxide (provided as 30% by weight hydrogen peroxide) as oxidant and acetone as solvent were mixed to form a liquid mixture which was fed from the bottom of the fixed bed reactor and flowed through the catalyst bed. Among them, the molar ratio of propylene to hydrogen peroxide is 2:1, the molar ratio of propylene to acetone is 1:10, and the weight hourly space velocity of propylene is 3h -1 . The catalyst bed was heated to a temperature of 40° C. by means of heating wires arranged in the catalyst bed. During the reaction, the heatin...
Embodiment 2
[0106] Adopt the same method as Example 1 to oxidize propylene, the difference is that the initial molar ratio of propylene to hydrogen peroxide is 2:1, during the reaction, when condition 1 is met for the first time, the Decrease the amount of heat exchange medium until condition 2 is met, stop reducing the amount of heat exchange medium and keep the amount of heat exchange medium; when condition 1 is met for the second time, increase the amount of liquid mixture by 0.02-2% / day The quality of hydrogen peroxide (realize by increasing the concentration of hydrogen peroxide in hydrogen peroxide, correspondingly reduce the consumption of hydrogen peroxide, so that the mol ratio of propylene and hydrogen peroxide remains constant) until condition 2 is satisfied, and so on (that is, in When condition 1 is met for the odd number of times, reduce the amount of heat exchange medium at the rate of 0.01-2% / day until condition 2 is met; The quality of hydrogen peroxide until condition 2)...
Embodiment 3
[0109]Adopt the method identical with embodiment 2 to oxidize propylene, difference is, also send into the ammoniacal liquor (concentration is 25% by weight) in the fixed-bed reactor, so that the pH value of the liquid mixture formed by propylene, hydrogen peroxide and acetone is adjusted to 8 . When the reaction lasted for 820 hours, the amount of the heat exchange medium was 75% of the initial amount, and the concentration of hydrogen peroxide in the hydrogen peroxide was 49% by weight. The results of the 0.5 hour and 820 hour reactions are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com