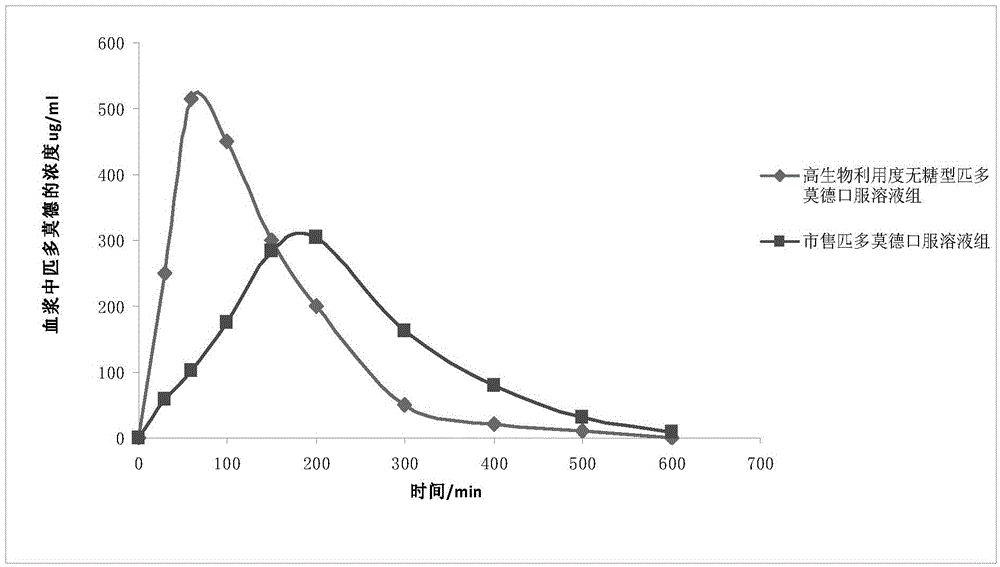
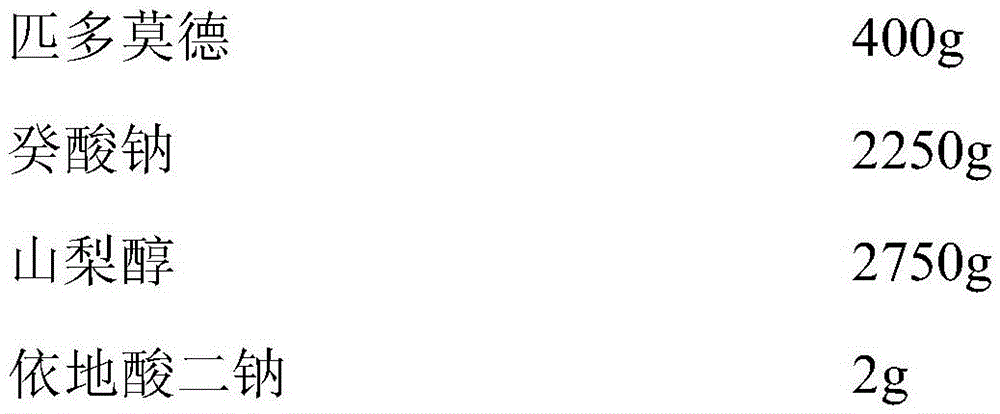High-bioavailability pidotimod oral liquid preparation and preparation method thereof
A technology for oral liquid and liquid preparation of domoda is applied in the field of oral liquid preparation of high bioavailability pidotimod and its preparation, which can solve the problems of low bioavailability and the like, achieve high blood drug concentration, stable storage, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
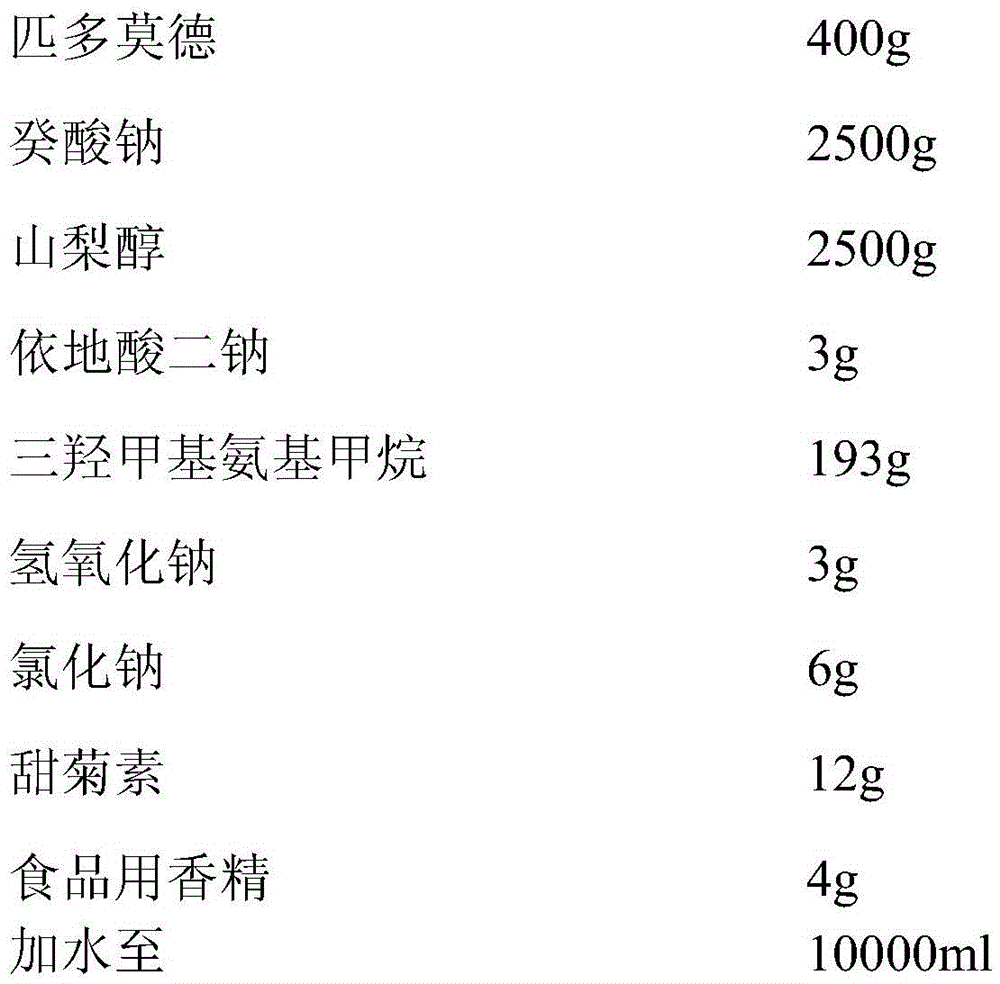
[0038] The oral liquid preparation of high bioavailability pidotimod of the present invention, prescription comprises,
[0039]
[0040] Add 50% of the prepared water, add 2500g of sodium caprate and 2500g of sorbitol and stir to dissolve, then add 400g of pidotimod, stir and dissolve, let stand for 10 minutes, then add 3g of disodium edetate, trimethylol Aminomethane 193g, sodium hydroxide 3g, sodium chloride 6g, stevioside 12g, food essence 4g, stir while adding, above-mentioned substances are fully dissolved, stir evenly, use 10wt% sodium hydroxide to fine-tune the pH value to 6.0-6.5, add purified water to 10000ml, stir well, filter with filter element, fill, and sterilize to prepare pidotimod oral liquid preparation with a specification of 0.4g / 10ml.
Embodiment 2
[0042] The oral liquid preparation of high bioavailability pidotimod of the present invention, prescription comprises,
[0043]
[0044]
[0045]Add 50% of the prepared water, add 2250 g of sodium caprate and 2750 g of sorbitol and stir to dissolve, then add 400 g of pidotimod, stir and dissolve, let stand for 15 minutes, then add 2 g of disodium edetate, 1 g of glycerin, Tris 200g, stevioside 3g, aspartame 5g, food essence 5g, stir while adding, the above-mentioned substances are fully dissolved, stir well, fine-tune the pH value to 7.0 with 10wt% sodium hydroxide ~7.5, add purified water to 10000ml, stir well, filter with a filter element, fill, and sterilize to prepare a pidotimod oral liquid preparation with a specification of 0.4g / 10ml.
Embodiment 3
[0047] The oral liquid preparation of high bioavailability pidotimod of the present invention, prescription comprises,
[0048]
[0049]
[0050] Add 50% of the prepared water, add sodium caprate 2000g, sorbitol 3000g to it and stir to dissolve, then add pidotimod 400g, stir and dissolve, let it stand for 15 minutes, then add calcium sodium edetate 2g, glycerin 1g, 180g of trishydroxymethylaminomethane, 6g of sodium hydroxide, 5g of sodium chloride, 10g of natural fruit flavor, 5g of essence for food, stir while adding, make the above-mentioned substances fully dissolve, stir evenly, add 10wt% sodium hydroxide Fine-tune the pH value to 6.0-6.5, add purified water to 10000ml, stir well, filter with a filter element, fill, and sterilize to prepare a pidotimod oral liquid preparation with a specification of 0.4g / 10ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com