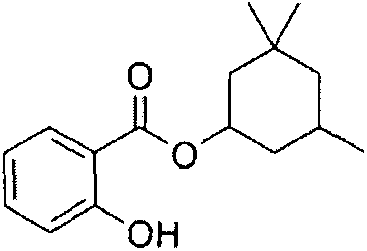
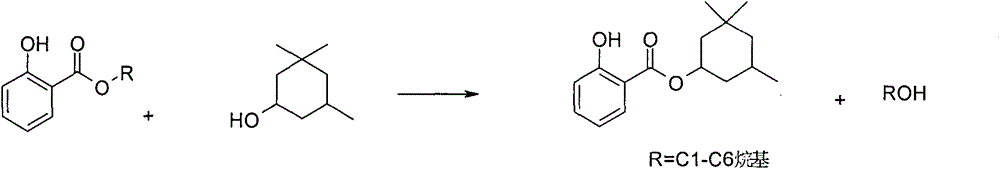Synthetic method of homosalate
A kind of technology of homosalate and synthesis method, applied in the synthesis field of ultraviolet absorber homosalate, to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 10.0g of methyl salicylate and 14.0g of 3,3,5-trimethylcyclohexanol to a 250ml three-necked flask, mix well, add 5.0g of potassium carbonate, increase the temperature to 120-130℃ and react for 10h, monitor the water by gas chromatography The conversion rate of methyl salicylate was 85.6%. Under reduced pressure distillation, 12.2 g of colorless and transparent humosalate was obtained, the yield was 70.8%, and the GC purity was greater than 99%.
Embodiment 2
[0024] Add 10.0g of methyl salicylate, 23.3g of 3,3,5-trimethylcyclohexanol, 0.50g of potassium carbonate, and 0.1g of tetrabutylammonium bromide into a 250ml three-necked flask. The temperature is raised to 180℃ and reacted for 13h. The conversion rate of methyl salicylate in the reaction system detected by chromatography was 97.2%. It was changed to a vacuum distillation device, and the front fraction was recovered for application to obtain 13.98 g of colorless and transparent humosalate with a yield of 81.1% and a GC purity of greater than 99%.
Embodiment 3
[0026] Add 10.0g of methyl salicylate, 18.7g of 3,3,5-trimethylcyclohexanol, 0.20g of sodium hydroxide, and 0.1g of potassium salicylate to a 250ml three-necked flask. The temperature is raised to 160℃ and the reaction is 8h. Gas chromatography Monitoring the conversion rate of methyl salicylate was 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com