Synthesis method based on diaminomaleonitrile asymmetric Schiff base
A technology of diaminomaleonitrile and synthetic method, which is applied in the field of synthesis of asymmetric Schiff base ligands based on diaminomaleonitrile, can solve the problem of low yield of Schiff base, lack of wide applicability, and solvent consumption Large and other problems, to achieve the effect of low cost, easy control of chemical components, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
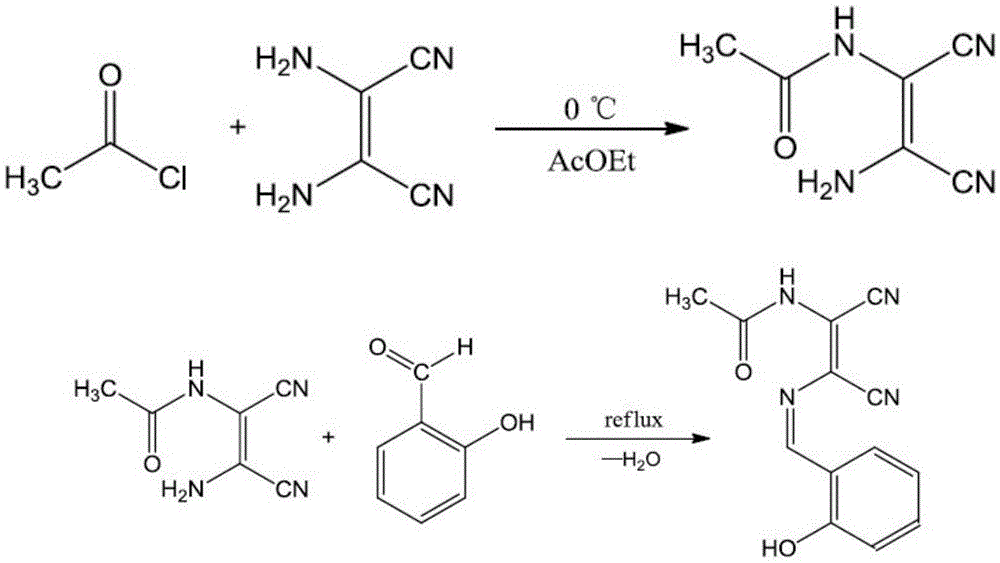
[0022] (1) Preparation of intermediate Ac-DAMN: Weigh 1.083g (10.0mmol) DAMN into a 50mL round bottom flask, add 10mL ethyl acetate, stir to dissolve. Then weigh 1.000g (13.0mmol) acetyl chloride in a 25mL beaker, add 5mL ethyl acetate to dissolve, transfer the reaction solution to a constant pressure dropping funnel, install the device, add acetyl chloride dropwise to DAMN, and react in ice Stirring was carried out in a water bath. During the reaction, the product gradually changed from brown to brownish yellow. The reaction product was filtered with suction, washed with 10 mL of ethyl acetate and 10 mL of diethyl ether, and dried to obtain wheat yellow powder Ac-DAMN.
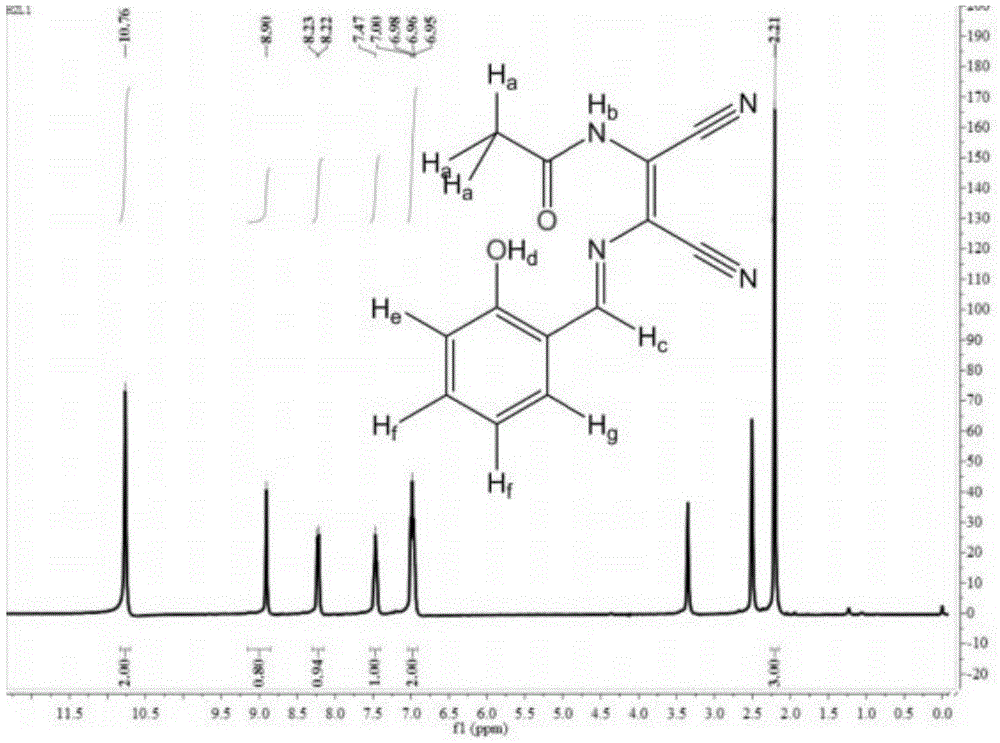
[0023] (2) Preparation of asymmetric Schiff base ligand salicylaldehyde acetylated diaminomaleonitrile: Weigh 1.500g (10.0mmol) Ac-DAMN in a 50mL round bottom flask, add 10mL absolute ethanol, stir, Let it dissolve. Then weigh 1.220g (10.0mmol) of salicylaldehyde in a 25mL beaker, add 10mL of absolute ethan...
Embodiment 2
[0025] Preparation of zinc complex: Weigh 0.025g (0.1mmol) L into a 25mL beaker, add 5mL of methanol, stir to dissolve, drop in 0.2mL of DMF, then add 1mL of 0.1mol / L NaOH methanol solution, stir for 0.5h, the ligand Completely dissolved, red. Then weigh 0.030g (0.1mmol) Zn(NO 3 ) 2 ·6H 2 O was dissolved in 4 mL of methanol solution in a 25 mL beaker, and this solution was added to the previous L solution, the color of the solution quickly changed to orange red, stirred for 0.5 h, and the solution remained clear. Filtrate, take the filtrate and let it stand at room temperature, and use the room temperature volatilization method for 5 days to precipitate small orange-yellow cube-shaped crystals.
[0026] X-ray single crystal diffraction test was carried out on the single crystal of the prepared asymmetric Schiff base zinc complex ( Figure 4 ) and thermogravimetric analysis ( Figure 5 ). Table 1 and Table 2 are the crystallographic data, structure revision data and parti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com