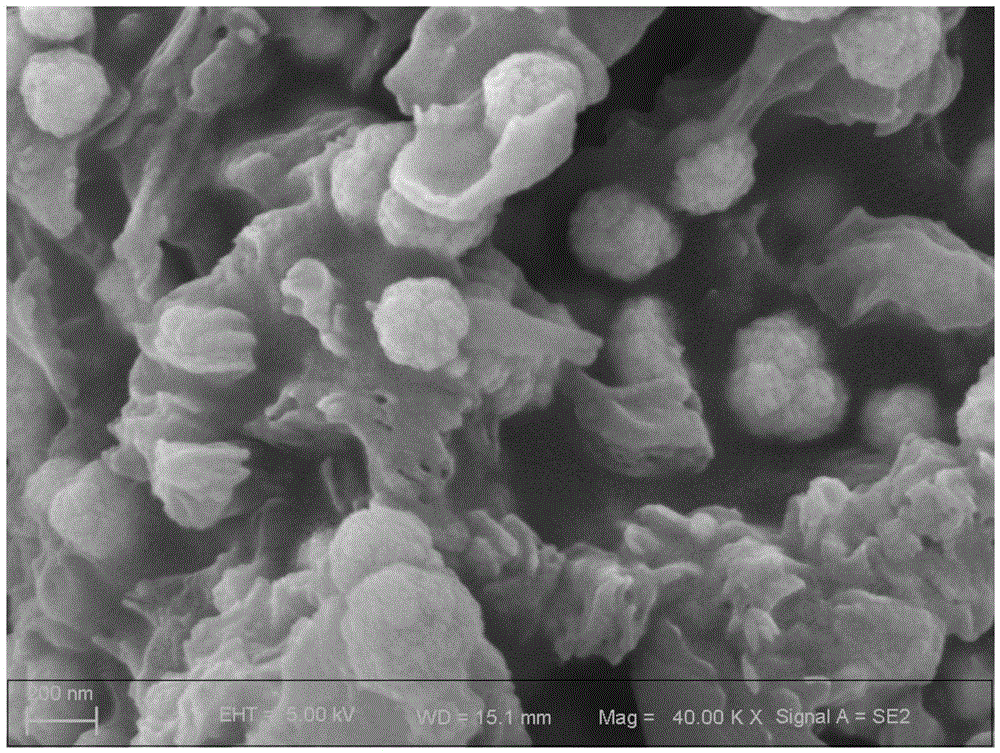
Method for preparing cadmium sulfide graphite-like carbon nitride compound photocatalyst
A graphite phase carbon nitride and photocatalyst technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of low photocatalytic efficiency, catalyst failure to achieve high-efficiency catalysis, and sunlight utilization Low rate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of graphite-like phase carbon nitride g-C by polycondensation method 3 N 4 catalyst:
[0029] 1) Put about 15g of urea in a crucible with a lid, and dry at 80°C for 4h.
[0030] 2) Move the crucible to the muffle furnace, set the heating rate to 10°C min -1 , Calcined at 550°C for 3h.
[0031] 3) After the calcination is completed, wait for the muffle furnace to drop to room temperature, collect the light yellow product in the crucible and grind it into a bag to obtain g-C 3 N 4 Catalyst for later use.
[0032] (2) Preparation of CdS / g-C by hydrothermal method 3 N 4 (1:1) composite photocatalyst:
[0033] 1), take 0.1g of g-C prepared above 3 N 4 The catalyst was placed in a 100 mL round bottom flask containing 30 mL of deionized water and stirred in an electric heating mantle for 1 h to obtain a suspension.
[0034] 2) Dissolve 7mL of 0.1M cadmium nitrate solution and 7mL of 0.15M thioacetamide solution (molar ratio Cd:S=1:1.5) into the above...
Embodiment 2
[0041] (1) Preparation of graphite-like phase carbon nitride g-C by polycondensation method 3 N 4 Catalyst is identical with embodiment 1.
[0042] (2) Preparation of CdS / g-C by hydrothermal method 3 N 4 (1:2) composite photocatalyst
[0043] 1), take 0.1g of g-C prepared above 3 N 4 The catalyst was placed in a 100 mL round bottom flask containing 30 mL of deionized water and stirred in an electric heating mantle for 1 h to obtain a suspension.
[0044] 2) Dissolve 3.5mL of 0.1M cadmium nitrate solution and 3.5mL of 0.15M thioacetamide solution (molar ratio Cd:S=1:1.5) into the suspension.
[0045] 3) Control the above reaction temperature at 90° C. and magnetically stir for 5 hours.
[0046] 4), the reaction is completed, and the mixture is transferred to a centrifuge tube for centrifugation after the round-bottomed flask is cooled to room temperature.
[0047] 5), the obtained product is washed several times with ethanol and deionized water.
[0048] 6) Place the p...
Embodiment 3
[0051] (1) Preparation of graphite-like phase carbon nitride g-C by polycondensation method 3 N 4 Catalyst is identical with embodiment 1.
[0052] (2) Preparation of CdS / g-C by hydrothermal method 3 N 4 (1:5) composite photocatalyst
[0053] 1), take 0.1g of g-C prepared above 3 N 4 The catalyst was placed in a 100 mL round bottom flask containing 30 mL of deionized water and stirred in an electric heating mantle for 1 h to obtain a suspension.
[0054]2) Dissolve 1.4mL of 0.1M cadmium nitrate solution and 1.4mL of 0.15M thioacetamide solution (molar ratio Cd:S=1:1.5) into the suspension.
[0055] 3) Control the above reaction temperature at 90° C. and magnetically stir for 5 hours.
[0056] 4), the reaction is completed, and the mixture is transferred to a centrifuge tube for centrifugation after the round bottom flask is cooled to room temperature.
[0057] 5), the obtained product is washed several times with ethanol and deionized water.
[0058] 6) Place the prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com