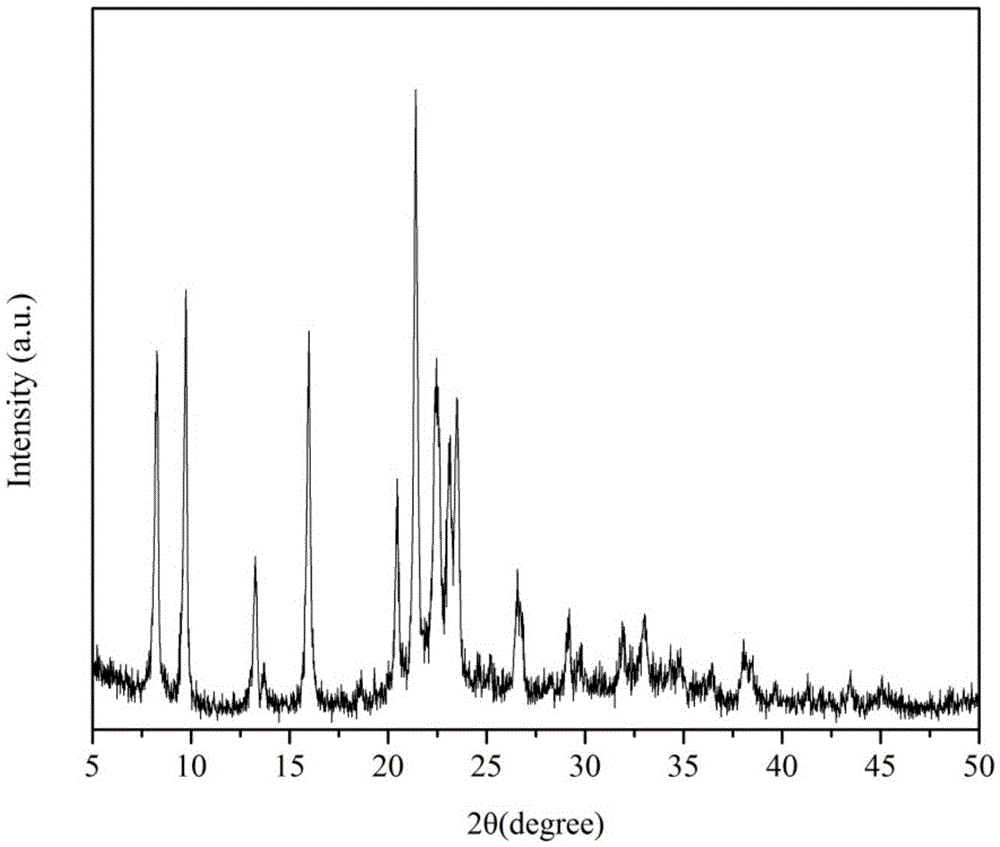
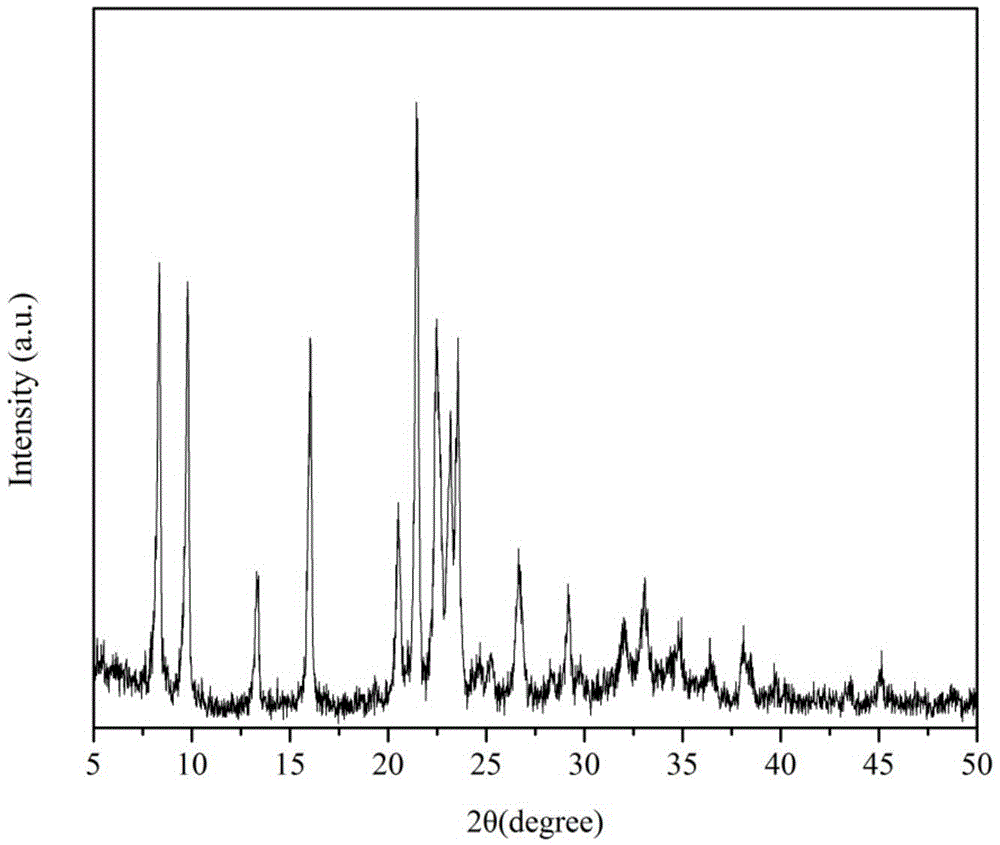
Method for using CuSAPO-11 molecular sieve for preparation of 2,6-dimethylnaphthalene
A technology for the catalytic preparation of dimethylnaphthalene, which is applied in the direction of molecular sieve catalysts, including molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of poor stability and high activity of 2,6-DMN selective catalysts, and achieve Ease of large-scale production, high reactivity, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of method utilizing CuSAPO-11 molecular sieve to catalyze the preparation of 2,6-dimethylnaphthalene in the present embodiment comprises the following steps:
[0036] 1) Preparation of CuSAPO-11 molecular sieve:
[0037] a. At 30°C, add aluminum isopropoxide and silica sol to 50% phosphoric acid aqueous solution, stir for 3 hours, then add di-n-propylamine and copper nitrate to the phosphoric acid aqueous solution, and stir for another 2 hours to obtain the reaction Mixture, the mol ratio of described aluminum isopropoxide, silica sol, phosphoric acid, di-n-propylamine and copper nitrate consumption is 0.5:0.05:0.6:0.1:0.05;
[0038] b. Sealing and crystallizing the reaction mixture prepared in step a in a polytetrafluoroethylene-lined stainless steel reactor, the crystallization temperature is 170°C, and the crystallization time is 24h;
[0039] c. Cooling the crystallized reactant in step b to room temperature, and repeatedly washing and separating with deioni...
Embodiment 2
[0048] A kind of method utilizing CuSAPO-11 molecular sieve to catalyze the preparation of 2,6-dimethylnaphthalene in the present embodiment comprises the following steps:
[0049] 1) Preparation of CuSAPO-11 molecular sieve:
[0050] a. At a temperature of 100°C, add aluminum isopropoxide and silica sol to an aqueous phosphoric acid solution with a concentration of 85%, stir for 3 hours, then add di-n-propylamine and copper nitrate to the aqueous phosphoric acid solution, and stir for another 2 hours to obtain a reaction Mixture, the mol ratio of described aluminum isopropoxide, silica sol, phosphoric acid, di-n-propylamine and copper nitrate consumption is 2:0.6:2.0:1.0:0.1;
[0051] b. Sealing and crystallizing the reaction mixture prepared in step a in a polytetrafluoroethylene-lined stainless steel reactor, the crystallization temperature is 200°C, and the crystallization time is 48h;
[0052] c. Cooling the crystallized reactant in step b to room temperature, and repeat...
Embodiment 3
[0061] A kind of method utilizing CuSAPO-11 molecular sieve to catalyze the preparation of 2,6-dimethylnaphthalene in the present embodiment comprises the following steps:
[0062] 1) Preparation of CuSAPO-11 molecular sieve:
[0063] a. At a temperature of 60°C, add aluminum isopropoxide and silica sol to a phosphoric acid aqueous solution with a concentration of 75%, stir for 3 hours, then add di-n-propylamine and copper nitrate to the phosphoric acid aqueous solution, and stir for another 2 hours to obtain a reaction Mixture, the mol ratio of described aluminum isopropoxide, silica sol, phosphoric acid, di-n-propylamine and copper nitrate consumption is 1.5:0.3:1:0.5:0.08;
[0064] b. Sealing and crystallizing the reaction mixture prepared in step a in a polytetrafluoroethylene-lined stainless steel reactor, the crystallization temperature is 180°C, and the crystallization time is 35h;
[0065] c. Cooling the crystallized reactant in step b to room temperature, and repeate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com