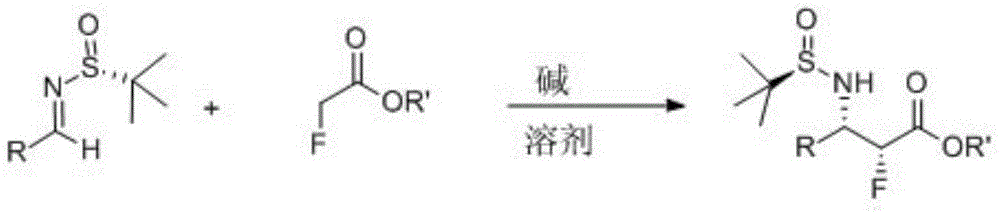
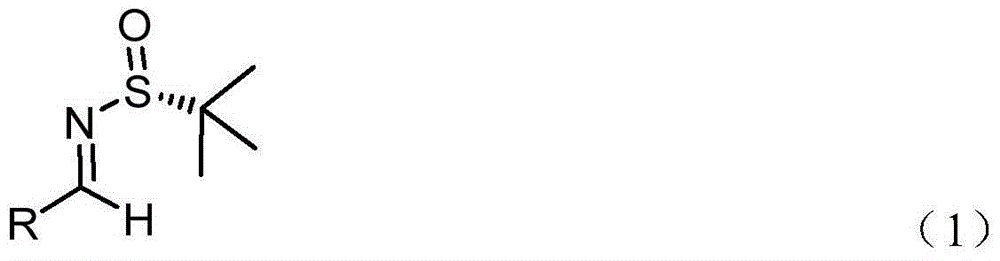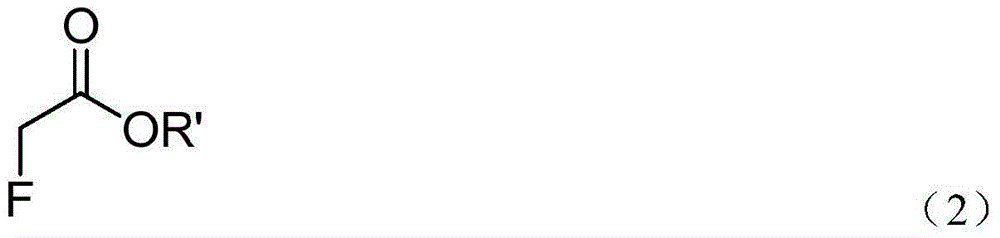Method for preparing chiral alpha-fluoro-beta-amino acid derivatives
An amino acid and derivative technology, applied in the field of preparing chiral α-fluoro-β-amino acid derivatives, can solve the problems of poor stereoselectivity, long route and expensive preparation method, and achieve high universality and mild process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At -80°C, LiHMDS (1.0 mL, 1.0 M dissolved in THF) was added dropwise containing methyl fluoroacetate (92 mg, 1.0 mmol), the imine represented by formula (2a) (209 mg, 1 mmol), And in a 3ml anhydrous THF reaction flask, the reaction system is protected by nitrogen. After the addition is complete, continue the low-temperature reaction for 0.5 hours. After the reaction was completed, 4ml of ammonium chloride aqueous solution was added to quench the reaction at low temperature. The reaction solution was transferred to a separatory funnel and extracted with ethyl acetate (10 ml×3). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Using ethyl acetate / petroleum ether (1:2) flash column chromatography, product 3a (265 mg) was obtained with a yield of 88% (265 mg) and a dr of 47:1.
[0030]
[0031] Compound 3 a Characterization data:
[0032]
[0033] Off-white to white crystalline solid, m.p.91.5-92.3℃; 1 HNMR(400M...
Embodiment 2
[0035] At -70℃, LiHMDS (1.4mL, 1.0M dissolved in THF) was added dropwise containing tert-butyl fluoroacetate (187 mg, 1.4 mmol) and the imine represented by formula (2a) (209 mg, 1 mmol) And in a 3ml ether reaction flask, the reaction system is protected by nitrogen. After the addition is complete, continue the low-temperature reaction for 0.5 hours. After the reaction was completed, 4ml of ammonium chloride aqueous solution was added to quench the reaction at low temperature. The reaction solution was transferred to a separatory funnel and extracted with ethyl acetate (10 ml×3). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Using ethyl acetate / petroleum ether (1:2) flash column chromatography, the product 3a' (281 mg) was obtained, the yield was 82% (281 mg), and the dr was 37:1.
[0036]
[0037] Characterization data of compound 3a:
[0038]
[0039] Off-white to white crystalline solid, m.p. 83.5-85.3℃; 1 HN...
Embodiment 3
[0041] At -20°C, NaHMDS (2.5 mL, 1.0M dissolved in THF) was added dropwise containing methyl fluoroacetate (184 mg, 2.0 mmol), the imine represented by formula (2b) (223 mg, 1 mmol) and In a 3ml dichloromethane reaction flask, the reaction system was protected with nitrogen. After the addition was completed, the reaction at low temperature was continued for 0.5 hours, and the reaction was quenched by adding 4 ml of ammonium chloride aqueous solution. The reaction solution was then transferred to a separatory funnel, and extracted with ethyl acetate (10 ml×3). After the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure. Using ethyl acetate / petroleum ether (1:2) flash column chromatography, product 3b was obtained with a yield of 80% (252 mg) and a dr of 15:1.
[0042]
[0043] Characterization data of compound 3b
[0044]
[0045] Off-white to white crystalline solid, m.p. 94.9-95.5℃; 1 HNMR(400MHz, CDCl 3 )δ7.80–6.24(m,4H), 5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com