Preparation method of high-purity topiramate
A topiramate and high-purity technology is applied in the field of preparation of high-purity topiramate, can solve problems such as unfavorable industrial production, reactor blockage, inconvenient operation, etc., and achieves the effects of less by-products, mild reaction, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
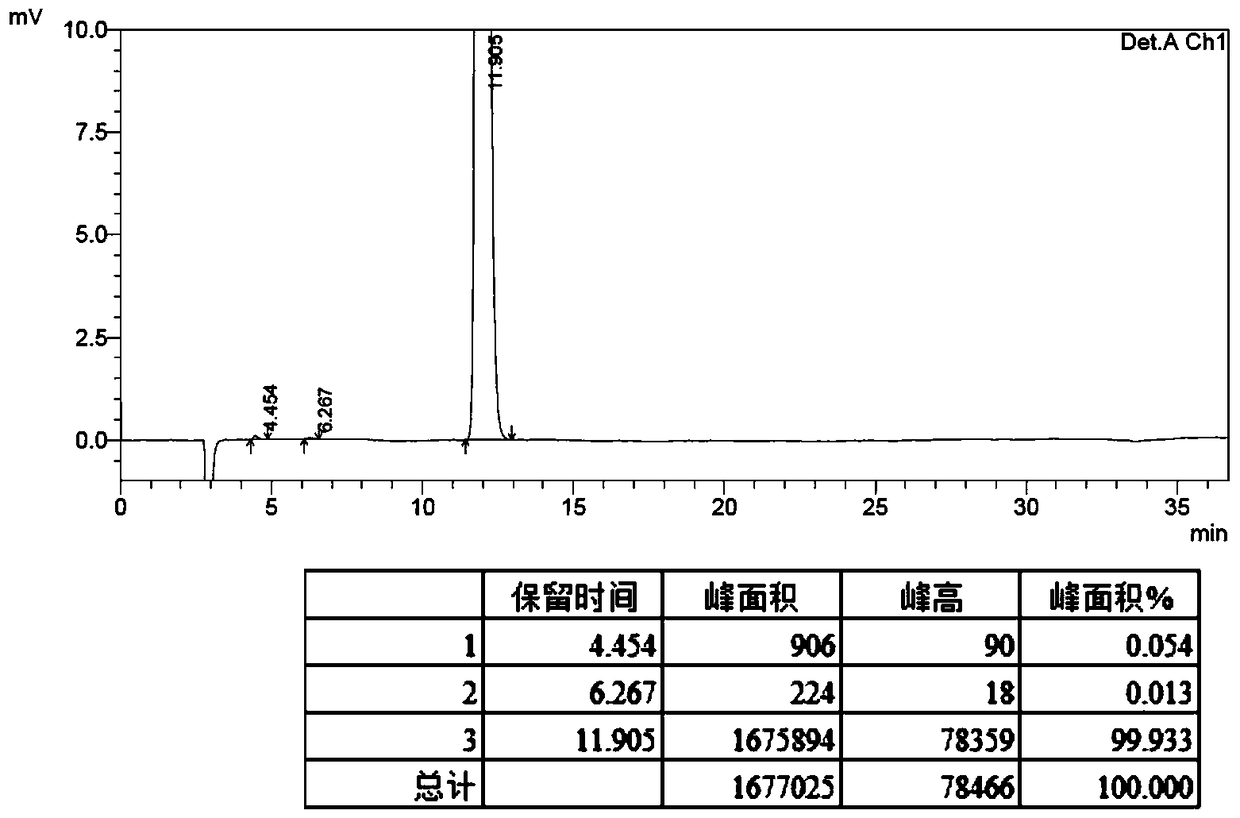
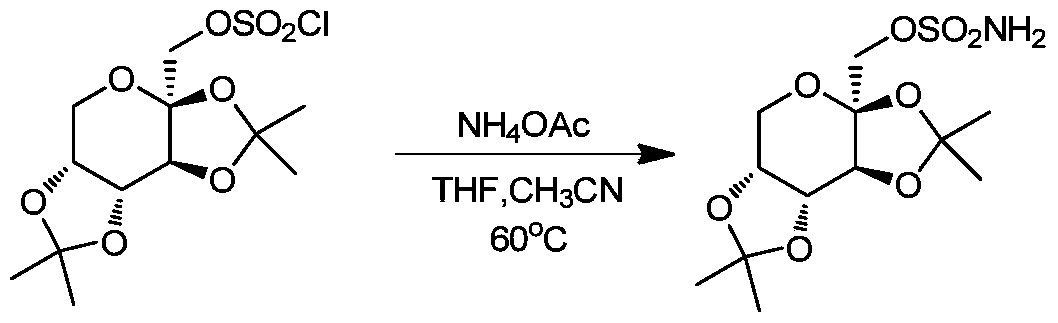
[0022] At room temperature, 2,3:4,5-bis-O-(1-methylethylene)-β-D-fructopyranose chlorosulfonate (15 g, 0.042 mmol) was dissolved in tetrahydrofuran ( 130 mL) and acetonitrile (13 mL), then added ammonium acetate (8.1 g, 0.105 mmol), heated to 60° C., and stirred for 3 hours. The reaction was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain the crude product as a pale yellow solid. The crude product of topiramate was recrystallized from n-hexane (45mL)-absolute ethanol (15mL), filtered with suction, and dried to obtain 9.66g of topiramate with an HPLC purity greater than 99%. The product recrystallized for the first time was recrystallized and refined again with n-hexane: anhydrous ethanol mixed solvent with a volume ratio of 3: 1 to obtain 8.67 g of topiramate with an HPLC purity greater than 99.5%.
[0023] 1 H NMR (400MHz, CDCl 3 )δ5.10 (s, 2H), 4.62 (dd, J = 7.9, 2.6Hz, 1H), 4.37-4.28 (m, 2H), 4.28-4.18 (m, 2H), 3.9...
Embodiment 2
[0025] At room temperature, 2,3:4,5-bis-O-(1-methylethylene)-β-D-fructopyranose chlorosulfonate (60 g, 0.167 mol) was dissolved in tetrahydrofuran ( 550 mL) and acetonitrile (50 mL), ammonium acetate (36.0 g, 0.468 mol) was added, heated to 60° C., and stirred for 3 hours. The reaction was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain the crude product as a pale yellow solid. The crude product of topiramate was recrystallized from n-hexane (180 mL)-absolute ethanol (60 mL), filtered with suction, and dried to obtain 40.34 g of topiramate with an HPLC purity greater than 99%. The product recrystallized for the first time was recrystallized and refined again with a mixed solvent of n-hexane: absolute ethanol with a volume ratio of 3:1 to obtain 35.79 g of topiramate, with an HPLC purity greater than 99.5%.
[0026] 1 H NMR (400MHz, CDCl 3 )δ5.10 (s, 2H), 4.62 (dd, J = 7.9, 2.6Hz, 1H), 4.37-4.28 (m, 2H), 4.28-4.18 (m, ...
Embodiment 3
[0028] At room temperature, 2,3:4,5-bis-O-(1-methylethylene)-β-D-fructopyranose chlorosulfonate (350 g, 0.978 mol) was dissolved in tetrahydrofuran ( 3000 mL) and acetonitrile (300 mL), then added ammonium acetate (226 g, 2.934 mol), heated to 60° C., and stirred for 4 hours. The reaction was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain the crude product as a pale yellow solid. The crude product of topiramate was recrystallized from n-hexane (1200mL)-absolute ethanol (400mL), filtered by suction and dried to obtain 225.37g of topiramate with an HPLC purity greater than 99%. The product recrystallized for the first time was recrystallized and refined again with n-hexane: anhydrous ethanol mixed solvent with a volume ratio of 3:1 to obtain 202.17 g of topiramate with an HPLC purity greater than 99.5%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ5.10 (s, 2H), 4.62 (dd, J = 7.9, 2.6Hz, 1H), 4.37-4.28 (m, 2H), 4.28-4.18 (m, 2H), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com