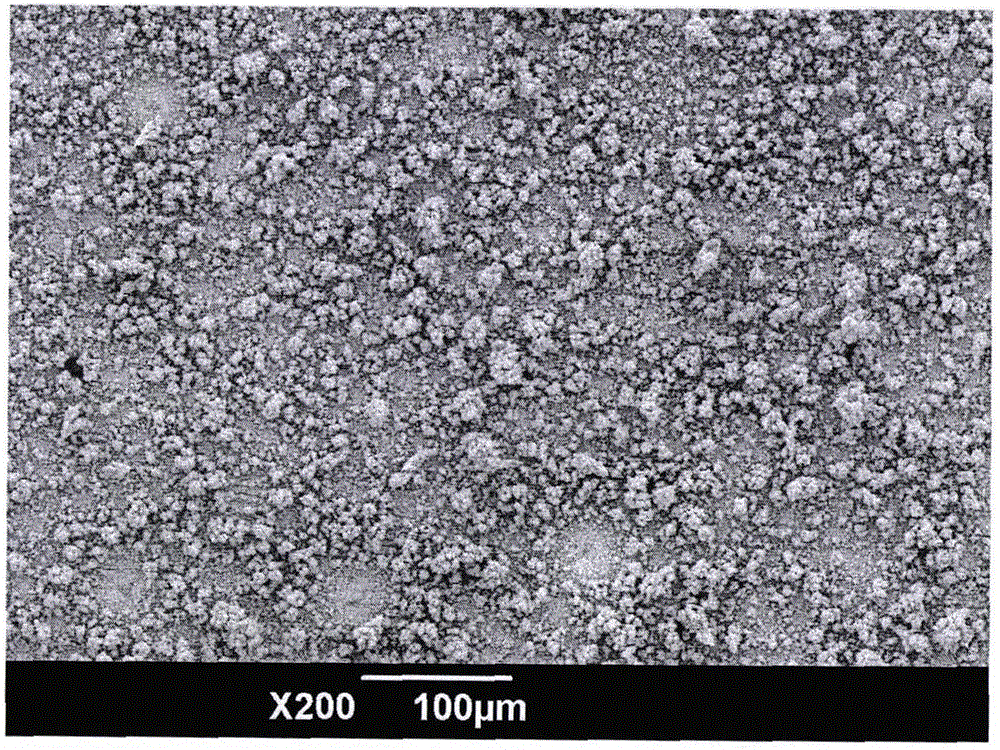
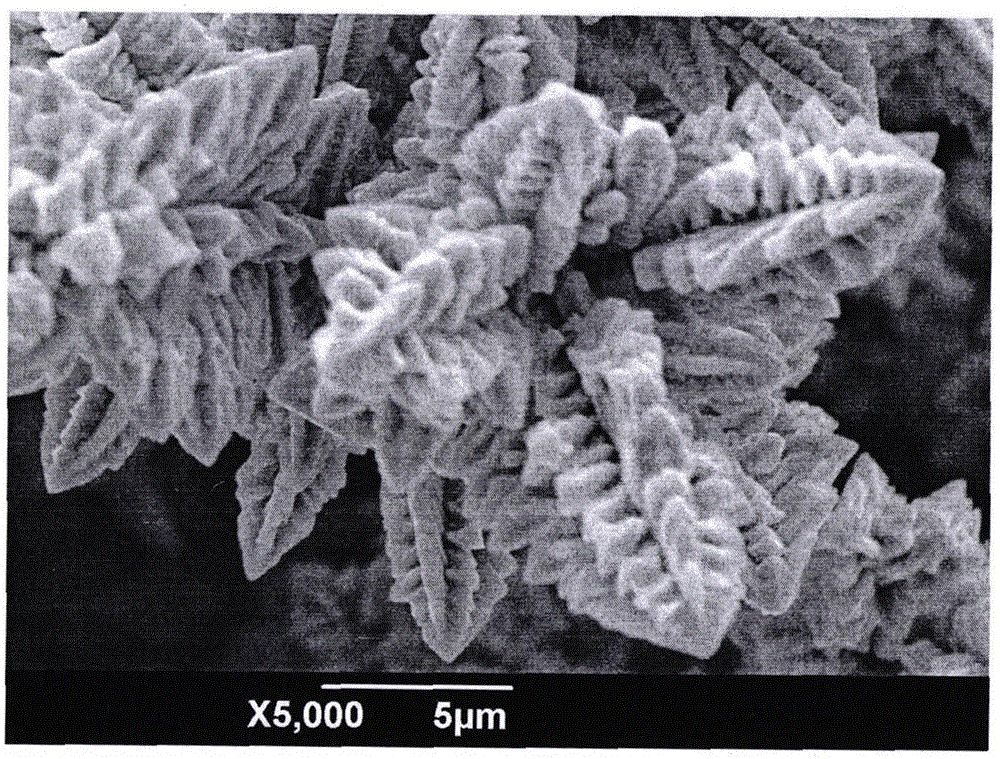
Electrochemical method for preparing super-hydrophobic surface of copper dendritic crystal
A super-hydrophobic surface and electrochemical technology, which is applied in the electrochemical field of preparing copper dendrite super-hydrophobic surface, can solve the problems of less research on super-hydrophobic surfaces, and achieve the effects of short preparation time, stable super-hydrophobic performance, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step 1. Slowly add 2.78ml of concentrated sulfuric acid to distilled water, then dissolve 3.75g of copper sulfate crystals in the solution, set the volume to 100mL, and stir well to obtain a copper sulfate concentration of 0.15mol / L and a sulfuric acid concentration of 0.5 mol / L solution A;
[0026] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.1 mol / L;
[0027] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0028] Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of t...
Embodiment 2
[0032] Step 1. Slowly add 2.78ml of concentrated sulfuric acid to distilled water, then dissolve 3.75g of copper sulfate crystals in the solution, set the volume to 100mL, and stir well to obtain a copper sulfate concentration of 0.15mol / L and a sulfuric acid concentration of 0.5 mol / L solution A;
[0033] Step 2. Dissolve 2.28 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.1 mol / L;
[0034] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0035] Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of t...
Embodiment 3
[0039] Step 1. Slowly add 16.68ml of concentrated sulfuric acid to distilled water, then dissolve 0.25g of copper sulfate crystals in the solution, set the volume to 100mL, and stir well to obtain a copper sulfate concentration of 0.01mol / L and a sulfuric acid concentration of 3mol / L solution A;
[0040] Step 2. Dissolve 1.14 g of myristic acid in absolute ethanol, set the volume to 100 mL, and stir evenly to obtain a solution B with a concentration of myristic acid of 0.05 mol / L;
[0041] Step 3: Polish two copper substrates with a size of 50mm×25mm×1.5mm with water sandpaper to remove the oxide layer on the surface of the copper substrate, then rinse the polished two copper substrates with distilled water and absolute ethanol in turn, blow dry for use;
[0042]Step 4. Put the solution A described in step 1 in the electrolytic cell as the electrolyte, insert the two copper substrates dried in step 3 into the electrolyte, and use them as the positive and negative poles of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com