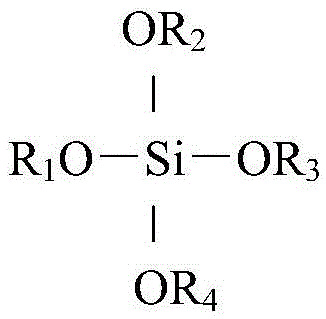Method for preparing 2-methyl-6-tert-butylnaphthalene from 2-methylnaphthalene through alkylation
A technology of methyldecalinyl and methylnaphthalene, which is applied in the field of 2-methylnaphthalene alkylation to prepare 2-methyl-6-tert-butylnaphthalene, which can solve the problems of large loss of carbon atoms and poor atom economy , to achieve the effect of reducing the loss of carbon atoms, easy separation, and inhibition of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Catalyst preparation
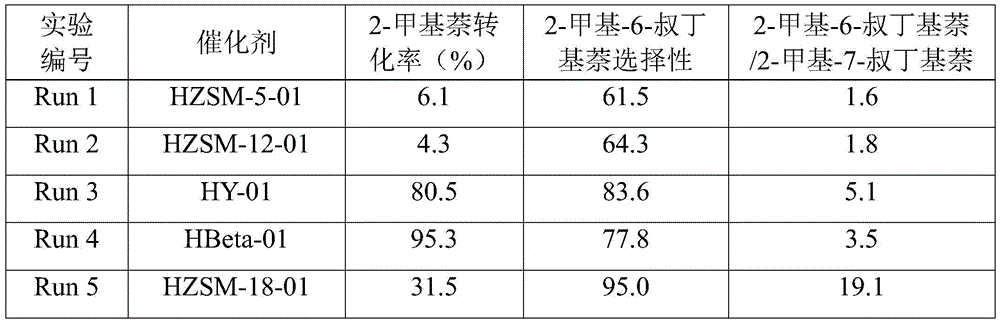
[0031] Roast 50g of HZSM-5, HZSM-12, HY, HBeta, HZSM-18, HMOR, SAPO-5 in a muffle furnace at 550°C for 4 hours, put them in 50g of tetramethyl silicate and soak them for 8 hours, and after centrifugation, The solid samples were dried in an air atmosphere at 120°C and calcined at 550°C for 3 hours to obtain modified molecular sieve catalysts, which were named HZSM-5-01, HZSM-12-01, HY-01, HBeta-01, and HZSM-18 -01, HMOR-01, SAPO-5-01.
Embodiment 2
[0033] Catalyst preparation
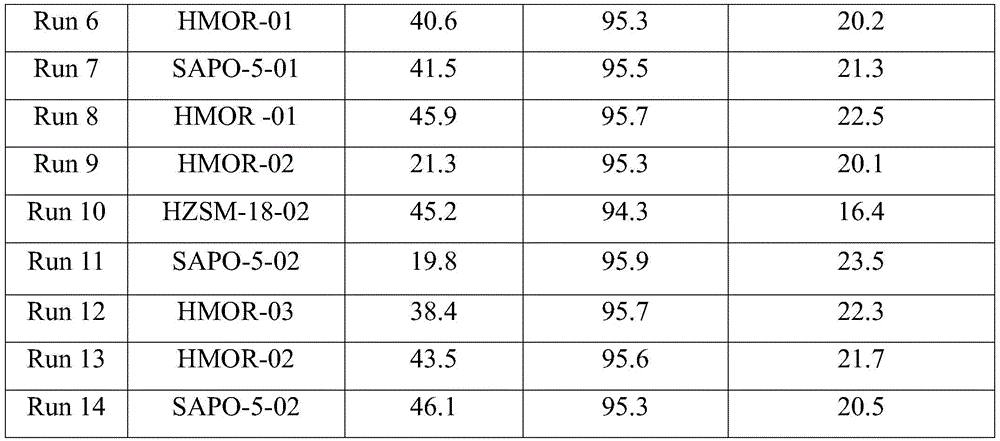
[0034] Roast 50g of HZSM-18, HMOR and SAPO-5 in a muffle furnace at 550°C for 4 hours, put them into 50g of tetraethyl silicate and soak them for 12 hours, after centrifugation, dry the solid samples in an air atmosphere at 120°C, 550°C ℃ for 4 hours to obtain modified molecular sieve catalysts, named HZSM-18-02, HMOR-02, SAPO-5-02, respectively.
Embodiment 3
[0036] Catalyst preparation
[0037] 50g of HMOR was roasted in a muffle furnace at 550°C for 4 hours, immersed in 50g of tetrapropyl silicate for 24 hours, centrifuged, the solid sample was dried in an air atmosphere at 120°C, and roasted at 550°C for 3 hours to obtain the modified The molecular sieve catalyst named HMOR-03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com