Preparation methods of dracorhodin and salt and intermediates thereof and intermediate compounds
A kind of compound, the technology of dracoidin, which is applied in the field of drexin preparation, can solve the problems of low total yield, poor selectivity, poor process reproducibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
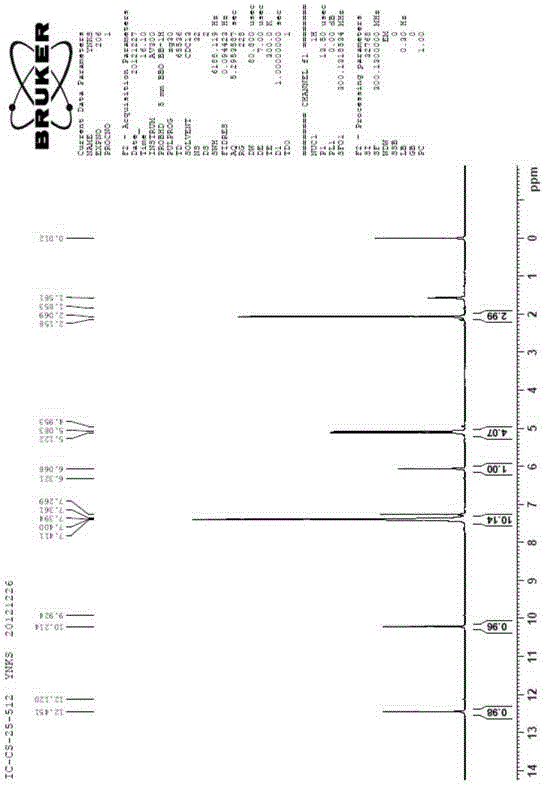
Image
Examples
preparation example Construction
[0121] As shown in the above reaction process (a), the preparation method of hematogenin provided by the present invention comprises the following steps:
[0122] 1) Vilsmeier formylation of compound SM and formylation reagent to produce compound I,
[0123]
[0124] 2) In solvent B, make compound I, 50-85wt% hydrazine hydrate and sodium hydroxide or potassium hydroxide undergo Huang Minglong reduction reaction, so that the aldehyde group of compound I is reduced to methyl, and compound II is obtained.
[0125]
[0126] 3) Vilsmeier formylation of compound II and formylation reagent to produce compound III,
[0127]
[0128] 4) In solvent D, under the protection of nitrogen, use a demethylation reagent to crack the methoxy group on the benzene ring of compound III to remove the methyl group to obtain compound IV,
[0129]
[0130] 5) Substituting the phenolic hydroxyl group of compound IV with benzyl halide in solvent E and in the presence of base E to obtain comp...
Embodiment 1
[0143] This example is used to illustrate the preparation method of hematine hydrochloride / perchlorate.
[0144] Step 1): preparation of compound Ⅰ (2,4,6-trimethoxybenzaldehyde)
[0145]
[0146] specific method:
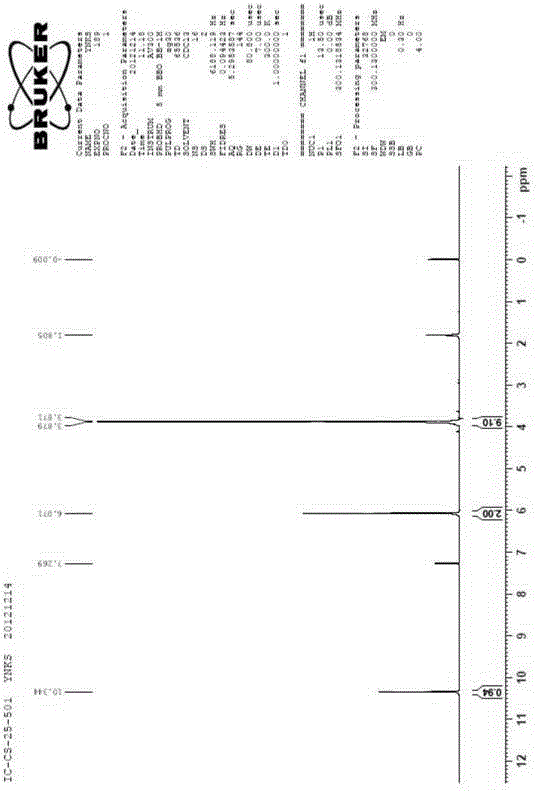
[0147] Mix 100g of compound SM (phloroglucinol trimethyl ether) and 500ml of DMF, cool the temperature in an ice-salt bath to below 0°C, add 201g (2.2eq) of phosphorus oxychloride dropwise, control the temperature below 0°C, and finish adding at below 0°C Insulation reaction for 1h, TLC tracking reaction completed; the reaction solution was poured into ice water, adjusted to weakly alkaline (pH 8-10) with potassium carbonate solution, crystallized overnight, suction filtered to obtain a white solid, and dried to obtain 101g of the product (compound Ⅰ), its hydrogen nuclear magnetic resonance (HNMR) spectrum is shown in figure 1 .
[0148] Compound Ⅰ proton magnetic spectrum (see figure 1 ) chemical shift data are: (CDCl3, 300M), 3.871-3.879, MeO, 9H; 6.071, ...
Embodiment 2
[0184] This example is used to illustrate the preparation method of heserodin perchlorate.
[0185] As shown in the following reaction formula, the perchlorate (compound Ⅸ) of hematine can be prepared from the hydrochloride (compound Ⅷ) according to the existing method.
[0186]
[0187] Therefore, in this embodiment, hematine hydrochloride can be prepared first by referring to steps 1)-8) of the embodiment, and then hematine perchlorate can be prepared according to the following step 9), the specific method is as follows:
[0188] specific method:
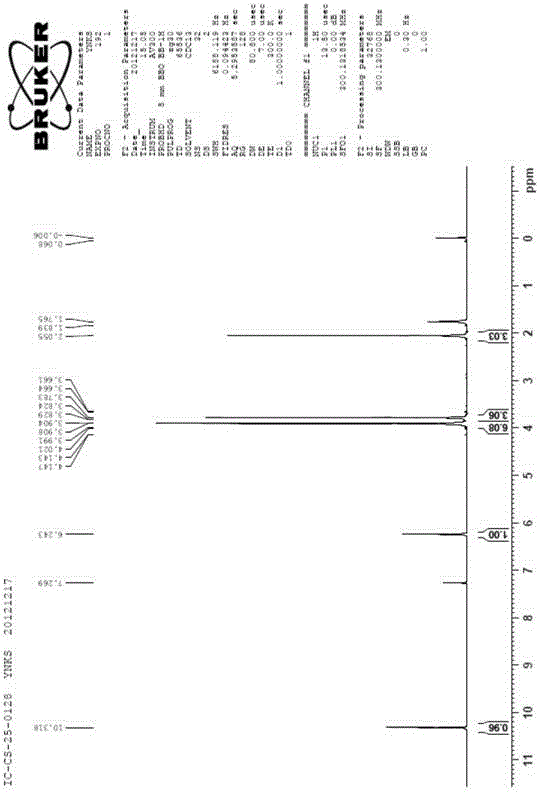
[0189] 5 g of compound VIII was dissolved in 60 ml of methanol, 80 ml of methanol solution of 2 ml of perchloric acid was added dropwise, and the reaction was stirred for 2 h. Precipitate orange-red crystals, filter, and recrystallize the solid with methanol to obtain 5g of compound IX pure product, its hydrogen nuclear magnetic resonance (HNMR) spectrum is shown in Figure 4 .
[0190] Compound Ⅸ proton magnetic spectrum (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com