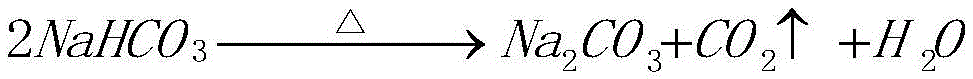Sodium nitrate preparation method
A technology of sodium nitrate and nitric acid, applied in directions such as the preparation of alkali metal nitrates, can solve the problems of high output and low cost of preparation, and achieve the effects of market competitiveness and economic benefits, reducing investment and improving economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
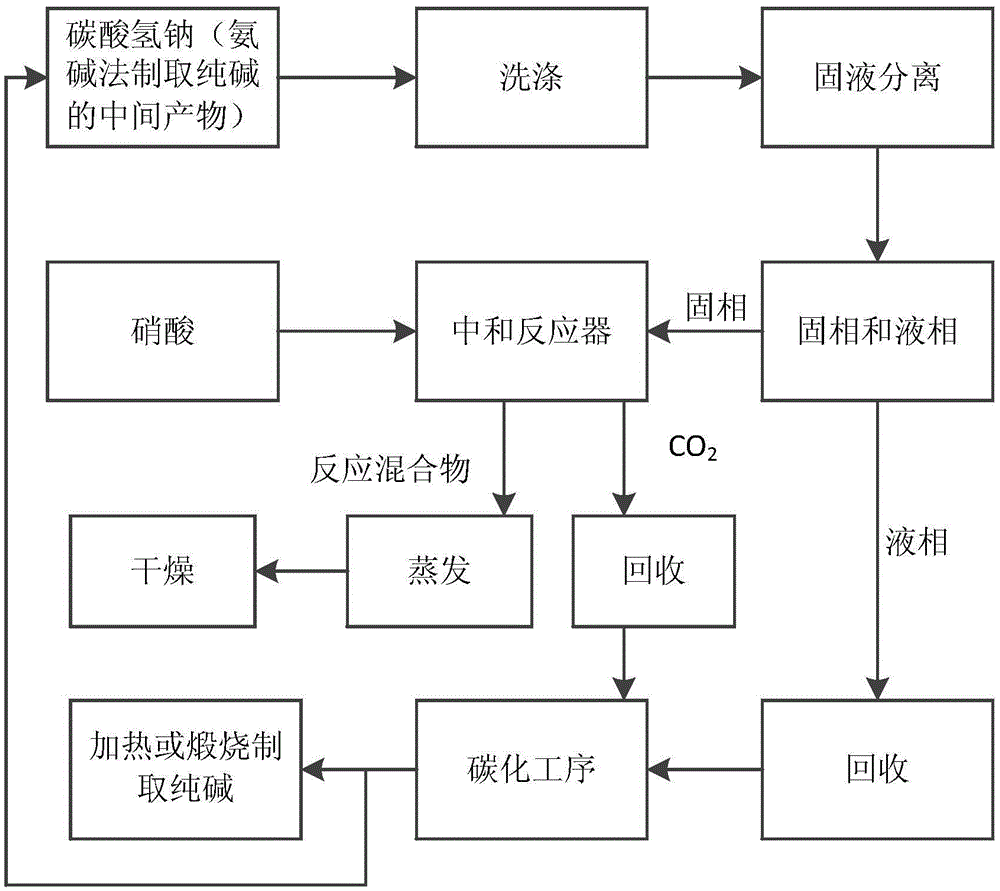
[0049] Such as figure 1 Shown, present embodiment adopts following steps to prepare sodium nitrate:
[0050] (1) The raw material sodium bicarbonate is beaten and washed with industrial water, and the amount of industrial water added is 0.58:1 according to the mass ratio of raw material sodium bicarbonate and industrial water. The washing slurry is filtered, and the liquid phase is the washing waste liquid, which can be transported to the carbonization process of producing soda ash by the ammonia-soda method, and used as a raw material for the carbonization process to dissolve sodium chloride; solid-phase drying to obtain purified sodium bicarbonate.
[0051] (2) Add a nitric acid solution with a mass fraction of 44.5% into the neutralization reactor by using a transfer pump, and turn on the stirring device in the neutralization reactor, and continuously stir the reaction solution in the neutralization reactor.
[0052] Afterwards, continue to add the solid-phase sodium bicar...
Embodiment 2
[0056] Present embodiment adopts following steps to prepare sodium nitrate:
[0057] (1) The raw material sodium bicarbonate is beaten and washed with industrial water, and the amount of industrial water added is 0.58:1 according to the mass ratio of raw material sodium bicarbonate and industrial water. The washing slurry is filtered, and the liquid phase is the washing waste liquid, which can be transported to the carbonization process of producing soda ash by the ammonia-soda method, and used as a raw material for the carbonization process to dissolve sodium chloride; solid-phase drying to obtain purified sodium bicarbonate.
[0058] (2) Add a nitric acid solution with a mass fraction of 44.5% into the neutralization reactor by using a transfer pump, and turn on the stirring device in the neutralization reactor to continuously stir the reaction solution in the neutralization reactor.
[0059] After that, continue to add solid-phase sodium bicarbonate to the neutralization re...
Embodiment 3
[0064] Produce sodium nitrate according to the step in above-mentioned embodiment two, but the pH value fine-tuning method of step (3) is slightly different with embodiment two, specifically: (a) at first detect whether there is CO in the neutralization reactor 2 Significant overflow; (b) in the absence of CO 2 In the case of obviously overflowing, add nitric acid in the neutralization reactor, the volume of nitric acid added is 0.6% of the volume of nitric acid in the original neutralization reactor, so that the pH value of the reaction solution in the neutralization reactor is 5; (c) Continue to add sodium carbonate to the neutralization reactor, during which there will be a small amount of CO 2 Gas is emitted until the pH value of the reaction solution is 6, and the addition is stopped, indicating that the reaction ends and reaches the end of the reaction.
[0065] The sodium nitrate finished product that present embodiment obtains is measured by the national standard test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com