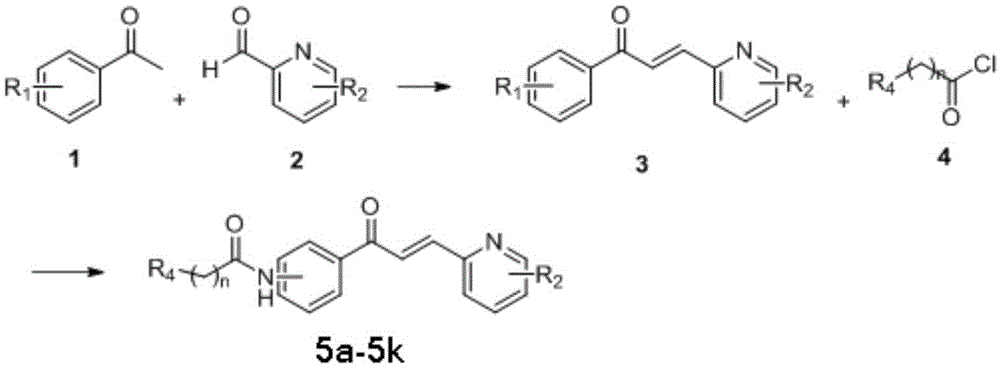
Chalcone derivatives with drug-resistant bacteria resistance activity
A technology of chalcone derivatives and drug-resistant bacteria, applied in antibacterial drugs, organic chemistry, etc., can solve the severe problems of antibiotic treatment and achieve good antibacterial effect and good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of compound 5a
[0036] Take sodium hydroxide (370mg, 9.25mmol) in a 250ml single-necked round bottom flask, add 50ml of water and dissolve under magnetic stirring at room temperature; then compound 1 (p-aminoacetophenone) (1.00g, 7.40mmol) and compound 2 (2 -Pyridine carboxaldehyde) (722ul, 7.58mmol) was added to a 100ml Erlenmeyer flask, 50ml of ethanol was added to ultrasound until the system was basically clear, and then the clear solution was added dropwise (1d / s) to the above-mentioned stirred sodium hydroxide solution In the single-neck bottle, the reaction continues at room temperature after the addition is completed, and the system is brown and clear at this time. After about 6h, TLC detection (PE:EA=1:1) showed that the reaction was complete. The reaction was stopped, and the reaction system was poured into about 50 ml of ice water. A large amount of yellow solid was precipitated immediately. The filter cake was washed with water to neutrality and va...
Embodiment 2
[0043] Preparation of compound 5b
[0044] Compound 1 is p-aminoacetophenone, compound 2 is 3-fluoro-2-pyridinecarboxaldehyde, and compound 4 is chloroacetyl chloride. The preparation method is the same as in Example 1.
[0045] The product is a pale yellow solid, the yield is 98.8%, m.p.: 157-158°C.
[0046] 1 HNMR(400MHz,CDCl3)δ8.43(d,J=4.4Hz,1H),8.39(s,1H),8.10(dd,J=24.8,12.0Hz,3H),7.98(dd,J=15.3,1.1 Hz, 1H), 7.66 (d, J = 8.7 Hz, 2H), 7.48-7.33 (m, 1H), 7.28 (dt, J = 8.5, 4.3 Hz, 1H), 4.16 (s, 2H).
[0047] 13 CNMR (101MHz, CDCl3) δ187.55,163.02,144.75,144.70,140.02,133.67,133.28,129.25,125.45,125.41,124.95,124.90,123.00,122.81,118.36,76.33,76.21,76.01,75.69,41.87,-0.00,- 1.03.
[0048] HR-MS(ESI)CalcdforC 16 H 13 ClFN 2 O 2 [M+H]+:319.0650,found:319.0648.
Embodiment 3
[0050] Preparation of compound 5c
[0051] Compound 1 is p-aminoacetophenone, compound 2 is 5-bromo-2-pyridinecarboxaldehyde, and compound 4 is chloroacetyl chloride. The preparation method is the same as in Example 1.
[0052] The product is a pale yellow solid, the yield is 85.3%, m.p.: 226-227°C.
[0053] 1 HNMR (400MHz, DMSO) δ 10.72 (s, 1H), 8.81 (d, J = 1.9 Hz, 1H), 8.23-8.08 (m, 4H), 7.91 (d, J = 8.4 Hz, 1H), 7.80 ( d, J = 8.6 Hz, 2H), 7.69 (d, J = 15.4 Hz, 1H), 4.32 (d, J = 6.3 Hz, 2H).
[0054] 13 CNMR(101MHz,DMSO)δ187.63,165.24,151.71,150.73,143.11,141.26,139.75,132.41,130.02,126.29,125.69,121.21,118.86,43.60,40.13,39.92,39.71,39.51,39.30,39.09,38.88.
[0055] HR-MS(ESI)CalcdforC 16 H 13 BrClN 2 O 2 [M+H] + :378.9849,found:378.9850.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com