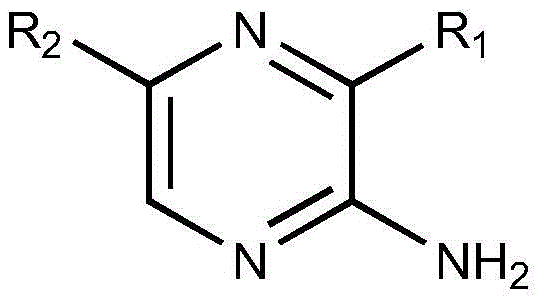
Preparation method of 2-aminopyrazine derivatives
A technology of aminopyrazine and derivatives, which is applied in the field of preparation of 2-aminopyrazine derivatives, can solve the problems of long route, heavy pollution, high cost, etc., and achieve the effects of high yield, cheap and easy-to-obtain raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
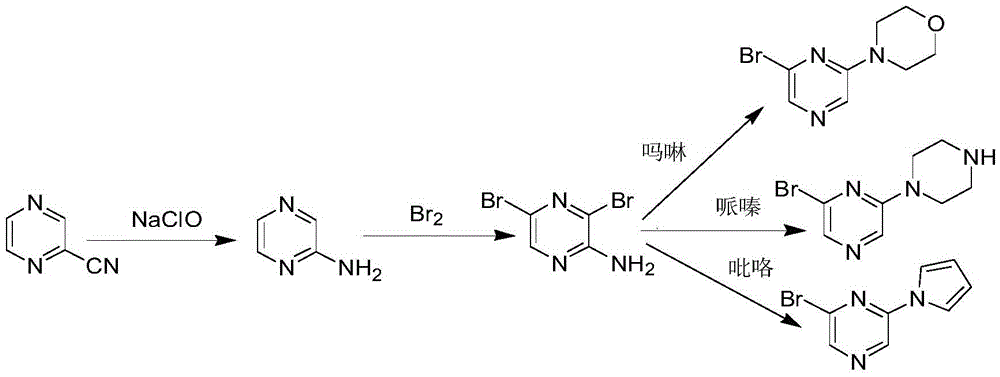
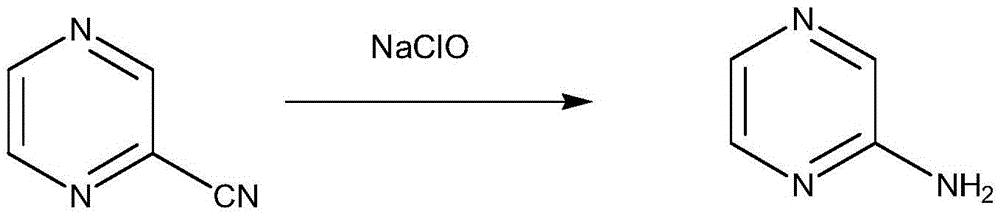
[0020] The preparation of embodiment 1,2-aminopyrazine
[0021]
[0022] In a 500ml three-neck flask, add 20% sodium hydroxide (30g) solution and sodium hypochlorite solution (100ml), add 2-cyanopyrazine (21g, 0.2mol) at room temperature, and stir for 1h. Then react at 50°C-60°C for 4h, extract with dichloromethane (4×200ml), dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 15.7g of white solid with a yield of 82.6%. Melting point 118-121°C; 1 H-NMR (CDCl 3 , 400MHz): δ7.72 (S, 1H, CH), 7.89 (S, 1H, CH), 7.91 (S, 1H, CH), 4.2 (S, 2H, NH2).
Embodiment 2
[0023] The preparation of embodiment 2,2-amino-3,5-dibromopyrazine
[0024]
[0025] In a 500ml three-necked flask, add a mixed solution of 2-aminopyrazine (19g, 0.2mol), dichloromethane (200ml) and pyridine (50ml), slowly add bromine (67.2g, 0.42mol) dichloro Methane (100ml) solution, stirred at room temperature for 4h, 100ml of water was added to the reaction system, stirred for 2h, the organic layer was washed with water (100ml×3), the organic phase was transferred to a flask equipped with silica gel and activated carbon, heated to reflux for 1h, and suction filtered , The solvent was distilled off under reduced pressure, and the resulting solid was added to n-hexane (45ml), refluxed for 2h and then filtered while hot. After drying, 39.4g of a yellow solid was obtained, with a yield of 77.8%. Melting point: 115-118°C; 1 H-NMR (CDCl 3 , 400MHz): δ7.91(S, 1H, CH), 4.2(S, 2H, NH 2 ).
Embodiment 3
[0026] The preparation of embodiment 3,2-amino-5-bromo-3-morpholinopyrazine
[0027]
[0028] In a 500ml three-necked flask, add morpholine (9.6g, 0.11mol), 2-amino-3,5-dibromopyrazine (25.3g, 0.1mol) and N-methylpyrrolidone (100ml), at 80°C The reaction was carried out for 6 hours, followed by TLC. After cooling to room temperature, 400 ml of water was added, stirred, and dried by suction filtration to obtain 22.5 g of a yellow solid with a yield of 86.7%. Melting point 134-137°C; 1 H-NMR (DMSO, 400MHz): δ7.70 (S, 1H), 6.28 (S, 2H), 3.85-3.62 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com