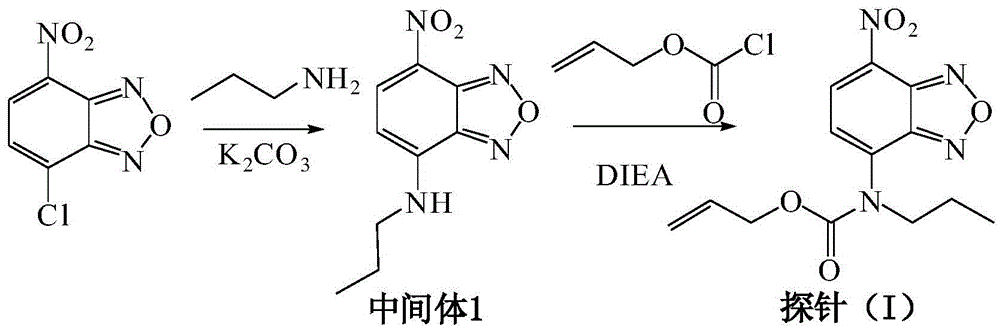
CO probe and preparation method and application thereof
A technology of probes and p-nitrobenzene, applied in chemical instruments and methods, analysis by making materials undergo chemical reactions, instruments, etc., can solve the problems of expensive instruments, complicated pre-treatment, inconvenient carrying, etc. Simple processing, easy to obtain products, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 200 mg of chlorop-nitrobenzofuroxan (1.0 mmol) to an acetonitrile solution (15 mL) containing 246L n-propylamine (3.0 mmol), add 300 mg of anhydrous potassium carbonate solid, and stir at room temperature for 1 hour. , Suction filtration, concentration of the filtrate, and column chromatography to obtain 4-propylamino-7-nitro-2,1,3-benzoxoxadiazole (Intermediate 1) with a yield of 64%;
[0033] (2) Dissolve 111mg of Intermediate 1 (0.5mmol) in 10mL of anhydrous acetonitrile, add 412μL of diisopropylethylamine (2.5mmol) under nitrogen protection, stir at room temperature for 0.5 hours, and then add 211μL of allyl chloroformate (2.0mmol) was slowly added dropwise to the above solution, after the dropwise addition was completed, reacted at room temperature for 12 hours, suction filtered, the filtrate was concentrated, and column chromatography was used to obtain 134mg of CO probe (I). The yield was 88%. The synthetic route of the product is as follows figure 1 Shown....
Embodiment 2
[0038] (1) Add 200 mg of chloro-p-nitrobenzofuran (1.0 mmol) to a tetrahydrofuran solution (15 mL) containing 247L of n-propylamine, add 280 mg of anhydrous potassium carbonate, stir at room temperature for 1 hour, and filter with suction. The filtrate Concentrate and column chromatography to obtain 4-propylamino-7-nitro-2,1,3-benzoxoxadiazole (Intermediate 1) with a yield of 60%;
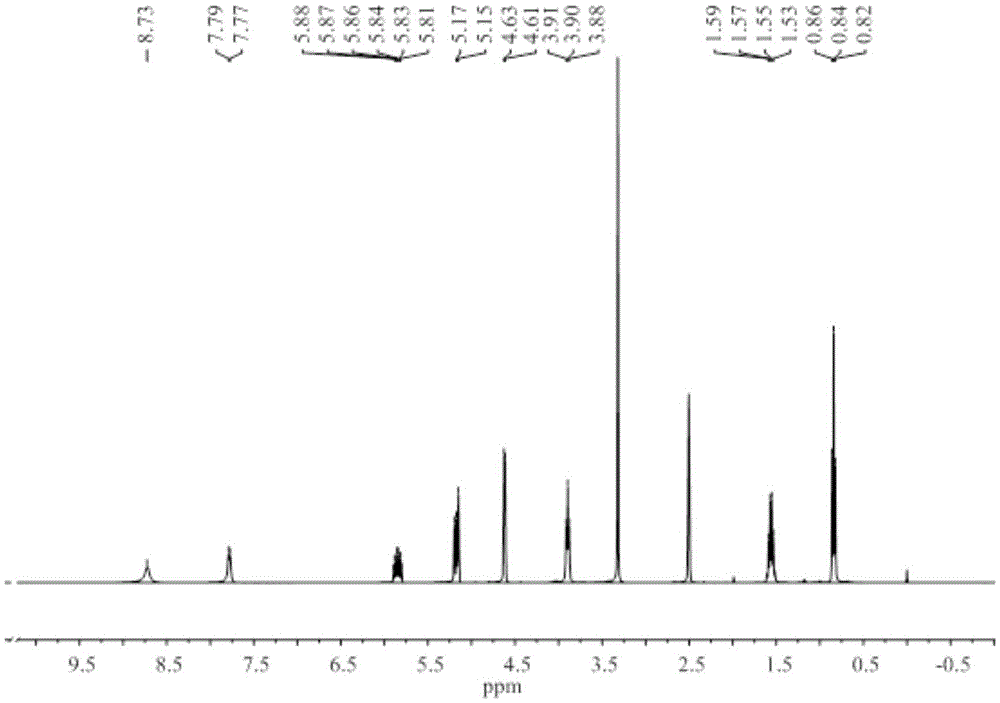
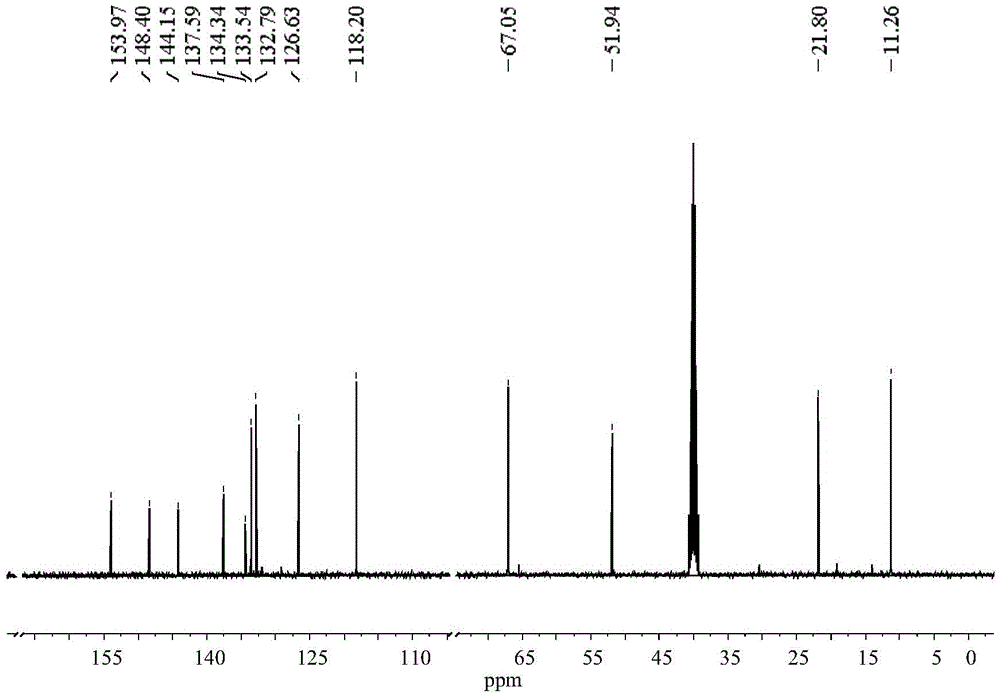
[0039] (2) Dissolve 112mg of Intermediate 1 (0.5mmol) in 10mL of tetrahydrofuran, add 330μL of diisopropylethylamine (2mmol) under nitrogen protection and stir at room temperature, then slowly add 317μL of allyl chloroformate (3.0mmol) Add dropwise to the above solution, after the dropwise addition, react overnight at room temperature, filter with suction, concentrate the filtrate, and column chromatography to obtain CO probe (I). The yield was 82%. product 1 HNMR spectrum and 13 CNMR spectrum and figure 2 with image 3 Consistent; the high-resolution mass spectrum of the product is consistent with ...
Embodiment 3
[0041] (1) Add 2.0g of chlorop-nitrobenzofurazan to a methanol solution (120mL) containing 2.5mL of n-propylamine, add 2.5g of anhydrous potassium carbonate solid, stir at room temperature for 2 hours, filter with suction, and concentrate the filtrate , Column chromatography to obtain 4-propylamino-7-nitro-2,1,3-benzoxoxadiazole (Intermediate 1) with a yield of 47%;
[0042] (2) Dissolve 1.2g of Intermediate 1 in 100mL of acetonitrile, add 3.2mL of diisopropylethylamine under the protection of nitrogen and stir at room temperature, then slowly drop 2.9mL of allyl chloroformate into the above solution, dropwise After the addition is complete, react overnight at room temperature, filter with suction, concentrate the filtrate, and column chromatography to obtain CO probe (I). The yield was 73%. product 1 HNMR spectrum and 13 CNMR spectrum and figure 2 with image 3 Consistent; the high-resolution mass spectrum of the product is consistent with Figure 4 Consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com